|
■ Bioresources information is available at the following URLs
|
 |
|
Research and Bioresources <No. 30> Genome editing for common marmosets
Erika Sasaki
· Director, Marmoset Research Department, Central Institute for Experimental Animals
· Professor (Special Appointment), Keio Advanced Research Centers, Keio University
Seven years ago (2009), I contributed an article to this newsletter reporting that by inducing the green fluorescent protein gene using a lentivirus vector in the embryo of a common marmoset (Callithrix jacchus), a small primate (hereinafter referred to as a marmoset), green transgenic marmosets were created [1].
Many useful genetically modified mouse models have been developed using target-gene knockout technology. Therefore, the development of a target-gene knockout model was considered necessary for marmosets. However, to create a model using target-gene knockout technology, embryonic-stem (ES) cells are essential, from which germ-line chimeras can be produced. Because ES cells with chimera-forming ability have not been established in animals other than mice and rats, target-gene knockout models for these animals could not be created. However, using genome-editing techniques, target-gene knockout models can be developed without using ES cells with chimera-forming ability. |
Development of immunodeficient marmosets using genome-editing techniques |
For a description of the usefulness and characteristics of marmosets as experimental animals, please refer to my previous article [1]. Our laboratory has been developing techniques to produce marmosets that can be used as human disease models using gene modification techniques. After establishing techniques to produce transgenic marmosets, we used various research methods to produce target-gene knockout marmosets. However, no useful results were obtained. A recent study reported that genome-editing techniques were useful for mammalian fertilized eggs, and thus we began to develop a target-gene knockout model from marmoset fertilized eggs using genome-editing techniques.
To develop genome-editing techniques for marmosets, we selected the interleukin-2 receptor common gamma chain (IL2rg) gene as the target gene.
We chose this gene for the following reasons: (1) the IL2rg gene exists on the X-chromosome, and a male marmoset develops immunodeficiency using only a mono-allelic knockout; (2) when an immunodeficient marmoset is produced, the produced marmoset can be used as a model in various research fields, such as regenerative medicine, hematology, oncology, and transplantation immunology, and (3) the produced marmoset develops immunodeficiency from its birth, and whether genome-editing techniques are effective for marmosets can be clarified during the early stage of growth.
|
For primates with long life cycles, it is preferable that the intended phenotype is expressed in the founder generation∗ 1 and that the modified gene is transmitted to the next generation. We aimed not only to knock out the target gene, but also to create an individual that was not in a mosaic state and in which the modified gene was uniformly distributed. If the modified gene in the individual is in a mosaic state and a wild-type gene remains, it is likely that the intended phenotype will not be expressed in the founder generation. Moreover, in experiments using primates, animal ethics must be taken into consideration and the development of animals in which modification of the target gene has failed should be prevented.
We then selected artificial nucleases with excellent cleavage efficiency using fibroblasts and established a method for estimating the acquisition ratio of knockout animals using marmoset fertilized eggs and in vitro genome editing to obtain the mosaic ratio of individuals (Fig. 1). The acquisition ratio and mosaic ratio estimated using this method were nearly correlated with or slightly lower than the actual creation ratio and the actual mosaic ratio of individuals, respectively. Currently, using this screening method, other genes can be successfully knocked out. Therefore, this screening method may be effective for genome editing in primates.
-----------------------------------------------------------------------
∗ 1 Founder generation: Generation of children of parents obtained by injecting a gene into a fertilized egg. F1 represents the children of founder individuals.
|
|
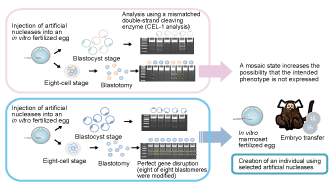
Fig. 1. Method for evaluating artificial nucleases in the creation of a target-gene knockout marmoset
Screening of artificial nucleases is the most important step of the in vitro development of a target-gene knockout marmoset that is not in a mosaic state. Upper panel: Artificial nucleases were injected into an in vitro fertilized egg, the egg was cultured up to the blastocyst stage, and gene modification was investigated by performing CEL-1 analysis; gene modification was confirmed in two of five blastocysts, and thus the ratio of knockout individuals was estimated to be 40% (upper part). Similarly, artificial nucleases were injected into an in vitro fertilized egg, the egg was cultured up to the eight-cell stage, blastomeres were separated from each other, and CEL-1 analysis was performed for each blastomere; modification was confirmed in three of eight blastomeres, and thus the modified gene was estimated to be in a mosaic state (lower part). Lower panel: By performing CEL-1 analysis, modification was confirmed in all fertilized eggs (upper part) and modification of the target gene was confirmed in all blastomeres, and thus the modified gene was estimated to not be in a mosaic state (lower part). Thus, by selecting appropriate artificial nucleases, target-gene knockout individuals expressing the intended phenotype can be obtained in the founder generation.
|
|
By injecting eHiFi-ZFN and platinum TALEN, which are artificial nucleases that target the IL2rg gene, into an in vitro marmoset fertilized egg in the one-cell stage, we created marmosets in which the IL2rg gene was knocked out and all cells possessed the same modified gene sequence (Fig. 2) [2]. In the cord blood of these individuals, T and NK cells were deleted, the number of B cells was normal and similar to that in human patients suffering from X-SCID∗ 2 (in mice, the number of B cells also markedly decreased), and the thymus gland was not present according to autopsy of deceased individuals. Therefore, these individuals were confirmed to be immunodeficient marmosets. Initially, these individuals were difficult to breed. However, because of improvements in anesthesia during Caesarean operation and oxygen concentration immediately after birth, the individuals could be bred for more than two years. When the sperm of a sexually mature male individual was investigated, the modified gene sequence, which was the same as that of somatic cells, was confirmed in the sperm. Therefore, germ-line chimeric marmosets can be produced.
-----------------------------------------------------------------------
∗ 2 X-SCID: X-linked severe combined immunodeficiency
|
In contrast, T cells, which were not confirmed to be present at birth, appeared, and B cells were deleted in adult individuals. In T cells, the number of which was increased, it was confirmed that the IL2rg gene was non-functional. Therefore, a signal transmission mechanism not involving the IL2rg gene may be related to the increased number of T cells. A similar phenomenon was reported in human patients suffering from X-SCID. The elucidation of the signal transmission mechanism related to the increased number of T cells is an interesting research theme that should be examined in future studies. IL2rg-knockout marmosets are useful as experimental models. |
|

Fig. 2. IL2rg-knockout marmoset developed using genome-editing techniques
Immunodeficient marmosets were created by knocking out the IL2rg gene. By improving the breeding conditions, these individuals could be bred for a long time (photographed by Kenya Sato). |
|
[1] |
Erika Sasaki: Use of common marmosets as experimental animals, BioResource now ! , 2009. 5(9). |
[2] |
Sato, K., et al., Cell Stem Cell, 2016. 19(1): p. 127-38.
|
|
|
|
How are bio-related tools developed ? |

Today, computers are indispensable in the study of biology.
Even if you only conduct wet research, you would have used tools such as Blast (1) and Primer 3 (2). This time, I would like to see behind the scenes of the development of these tools.

Open source software
Open source software is literally software in which source code (text written in program language) is disclosed. Disclosing the program has its own merits in that software users can correct bugs and give back to creators. Many software programs exist, of which the sources are disclosed also in bio-related tools. For example, TopHat (3), introduced last time, also has the source code released.
|

GitHub
GitHub is a shared web service that uses the software development project version control system (Git), which allows you to browse through the source code and manage bugs. Below is the GitHub page of TopHat (Fig. 1).
[Author or organization name / project name] is displayed on the upper left of the page (Fig. 1 A).
Further, several statuses are displayed under the project description (Fig. 1 B)
″Commits″ is the number of changes made to the project. As you can see, it has been updated many times to improve the program. ″Releases″ allows you to download programs of any timing (Figure 2). Reproducibility of analysis using the tool is important; so do describe the version. In addition, ″contributors″ are people contributing to the project. TopHat seems to have been changed 4,346 times by 5 developers (as of 2016.09.01).
By clicking on the colored part (Fig. 1 B) you can see the percentage of the programming language used in the project. TopHat mainly turned out to be written in C ++ (Fig. 3).

Let us report bugs
GitHub is not a service only for developers. Users can also contribute to improving the tool. By clicking the ″issues″ tab (Fig. 1 C) on the project page, you can see a list of currently reported problems and requests. Click on Issue in the list to see the exchange of comments (Fig. 4). If you report a bug, there may be a reply from the developer, as shown in Fig. 4. You can also register a new Issue from the ″new issue″ button. To register Issue, you need to create a GitHub account.
When registering a bug using Issue, try to describe information and logs that can reproduce bugs, such as OS and program version, to help developers.
In addition, there may be rules to report; so look at the README of the project beforehand. If there is a README file in the root folder of the project, the README is displayed at the bottom of the GitHub page (Fig. 1). |
|
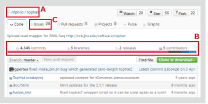
Fig. 1. GitHub page of TopHat
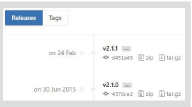
Fig. 2. Releases list

Fig. 3. Percentage of language used in the project
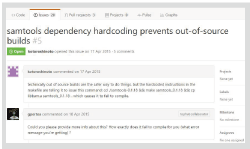
Fig. 4. Issue |
In this issue, I introduced GitHub, which is often used behind the scenes of the development stage.
GitHub is a tool that connects developers and users. If the tools you usually use are developed regularly on GitHub, why not check it out.
(Shunsuke Maeda) |
|
|