|
 |
■Bioresources information is available at the following URLs
|
 |
|
Development of the Ultra-Superovulation Induction Method for Mice
Naomi Nakagata (Professor, Division of Reproductive Engineering,
Center for Animal Resources and Development,
Institute of Resource Development and Analysis,
Kumamoto University)
For two years (2012–2013), our research was adopted by the National BioResearch Project Fundamental Technologies Upgrading Program (Focus: Establishment of in vitro fertilization systems for all mouse strains). During this time, we worked to develop technologies for in vitro fertilization systems for all mouse strains, using cryopreserved sperm, sperm obtained through refrigerated transportation, and low-fertility fresh sperm. Consequently, we succeeded in establishing in vitro fertilization systems that allowed us to obtain extremely high fertility rates by using all possible combinations of frozen, chilled, and low-fertility fresh sperm and fresh and frozen unfertilized eggs. However, from the success of this research project, another problem emerged: the need to obtain a large number of mature ova from each female mouse.
When the previously developed superovulation induction method was used, approximately 20 ova could only be ovulated by a female mouse on average, although several million sperm could be obtained from each male mouse. If a female mouse ovulates 20 ova, only a maximum of 20 fertilized eggs can be obtained through in vitro fertilization from a pair of male and female mice. If a female mouse could ovulate 100 ova, it would be possible to obtain 100 fertilized eggs from a pair of male and female mice.
|
The conventional superovulation induction method for mice involves administering pregnant mare serum gonadotropin (PMSG: a follicle stimulating (FSH)-like hormone), to grow a large number of follicles. However, since the secretion of FSH is inhibited by inhibin released by the growing follicles, the number of ova that a female mouse ovulates can never be more than about 30.
We therefore neutralized inhibin in the body of a female mouse by using an anti-inhibin antibody and simultaneously administered PMSG to grow a large number of follicles. This synergic effect enabled us to successfully obtain more than 100 ova from a single female mouse (Fig. 1: the ultra-superovulation induction method), which represented 3−4 times more ova than could have been obtained using the conventional method. Moreover, we have confirmed that both the fertility of the obtained ova and their developmental potency (for creating newborn babies) are normal (Fig. 2 and Fig. 3) (Reference). |
|
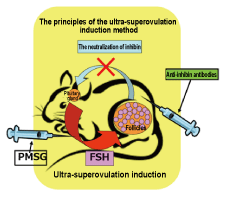
Fig. 1. The ultra-superovulation induction method |
|
| |

Fig. 2. Two-cell embryos obtained by performing in vitro fertilization using ova ovulated by a female mouse |
|

Fig. 3. Newborn babies obtained by transplanting two-cell embryos obtained from the ova of a female mouse into provisional parent oviducts |
|
The economic impact of the ultra-superovulation induction method is described below.
(1) Decrease in the number of female animals needed
This method requires only 1/3−1/4 of the number of female animals that would be needed to collect ova using the conventional method. This significant reduction in the number of experimental animals represents an important advance in animal protection and welfare. In other words, this reduction is extremely meaningful, not only from the perspective of live science research, but also from the perspective of social responsibility for animal protection and welfare. A method that can reduce the number of experimental animals needed for a range of different research activities is sure to be highly valued worldwide.
(2) Efficient production, collection, preservation, and distribution of genetically modified mice
When a large number of ova can be ovulated by a small number of female mice, in vitro fertilization and embryo transplantation can be carried out easily. As a result, genetically modified mice can be efficiently collected, preserved, and distributed.
(3) Application to other animal species
The ultra-superovulation induction method is highly applicable to rats, hamsters, and guinea pigs, species for which no other method has been established. The use of this method will therefore be extended.
The Center for Animal Resources and Development (CARD) at the Kumamoto University Institute of Resource Development and Analysis has developed and modified various reproductive engineering technologies and attempted to improve the efficiency of the mouse bank system. The CARD mouse embryo/sperm bank system consists of a “deposition and supply system” and a “private bank system.” In the deposition and supply system, individual mice, embryos, and sperm are deposited and preserved. Information on the preserved resources is exhibited, and available resources are provided to third parties. All of the deposition costs, including transportation to and cryopreservation at CARD are free of charge. It is possible to deposit mouse resources with particular conditions, ensuring the following: that only the results of joint research can be published on websites; the supply is limited to joint research projects; the supply is limited to particular research purposes; the consent of the establisher is required; and no distribution is allowed during a certain period. There is a charge for using the supply (actual expenses). Having reviewed the information on our website, an individual must first obtain a letter of consent from the depositor, and then apply to CARD for access to the supply of individuals, embryos, or sperm.
The private bank system cryopreserves mouse embryos and sperm deposited by clients in return for payment, on condition that the cryopreserved embryos and sperm are not distributed to third parties and that no information about the cryopreserved embryos or sperm is made publicly available. This system also produces individuals from the cryopreserved embryos and sperm and supplies the produced individuals exclusively to clients.
2,000 mouse strains have already been deposited in this deposition and supply system; more than 700 individual mice, embryos, and sperm have been supplied to researchers. The number of domestic and foreign research institutions using this resource has increased.
Although the private bank system was launched in fiscal year 2006, this system already holds the cryopreserved embryos and sperm of approximately 900 mouse strains. More than 700 requests have been made for mice produced using these cryopreserved embryos and sperm. It is clear that this system is making steady progress.
|
In the future, we will use our newly developed ultra-superovulation induction method to improve the efficiency of the mouse bank system in various ways. We also hope that many other researchers will make use of the mouse bank. This method, together with other reproductive engineering technologies, is described and promoted in online manuals※.
We have held 50 training courses on reproductive engineering technologies, including in vitro fertilization, the cryopreservation of embryos and sperm, and embryo transplantation, both in Japan and internationally. In June 2016, we will hold a training course at the Pasteur Institute in Paris. We are committed to internationally standardizing reproductive engineering technologies.
|
| |
Reference |
| |
Takeo et al. Superovulation Using the Combined Administration of Inhibin Antiserum and Equine Chorionic Gonadotropin Increases the Number of Ovulated Oocytes in C57BL/6 Female Mice. PLoS One 2015;10(5):e0128330. |
|
|
|
Introducing New Features of Windows 10
|

On July 29, 2015, Windows 10, the latest Windows operating system, was released. You can upgrade to Windows 10 from Windows 8.1 and Windows 7 for free. In this article, I will introduce two new productivity features that have been added to Windows 10.
|

Task View
When you have multiple applications running simultaneously, this feature allows you to switch between applications that are hiding behind other windows or are minimized. By clicking on the Task View icon located on the status bar, running applications will be shown as a list of thumbnail images (Fig. 1). By clicking on a thumbnail, you can bring that application to the foreground. Multiple windows of an application will also be displayed as separate thumbnails, making it easy to switch to a particular window by looking at its thumbnail.
|
|
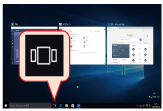
Fig. 1. The Task View.
|
|

Virtual Desktop
| This new feature allows you to create desktops in a virtual environment and switch between them. This is a convenient feature for performing multiple tasks simultaneously. For example, suppose you are performing the following tasks at the same time: |
| |
● |
Creating presentation slides while reading a journal article |
| |
● |
Writing a document while browsing for information on the Web |
| Since four applications with different objectives are running at the same time, there may be confusion when trying to switch between them. To resolve this issue, you can assign applications to different virtual desktops based on associated tasks: |
| |
● |
PDF viewer and PowerPoint |
| |
● |
Internet browser and Word |
This enables you to switch between tasks efficiently, simply by switching desktops. Instructions for using this feature are provided below.
|
|

Clicking on the Task View icon and then clicking Add a Desktop will add a new desktop, which will be shown at the bottom of the screen (Fig. 2). You can switch to the new desktop by clicking on its image. If you launch an application on a virtual desktop, it will only be displayed on that desktop. If you wish to move an application that is already running to a different desktop, show the Task View, and move the cursor over the desktop where the application is running. Next, right-click the application’s thumbnail from the list, select Move and specify the destination desktop of choice (Fig. 3). |
| |
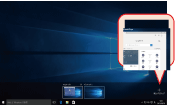
Fig. 2. Adding a new virtual desktop.
|
|
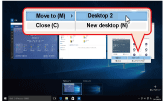
Fig. 3. Moving an application between virtual desktops.
|
|

The features introduced in this article are particularly useful on notebook PCs where the screen display area is limited. These features have been available on Mac OS X and certain Linux OS distributions; their absence was inconvenient when I switched from Mac OS X to Windows 7. I believe that the additional features on Windows 10 provide a broader choice of operating systems.
(Shunsuke Maeda) |
|
|