Research and Bioresources <No.21> Investigation of the Mechanism underlying Three-dimensional Organogenesis by Isolating Flat Medaka Mutants!
Hiroshi Nishina (Professor, Medical Research Institute,
Tokyo Medical and Dental University)
A century-old theory by D'Arcy Thompson, a British mathematical biologist, states that the shapes of organisms on the earth greatly reflect the influence of gravity on them. However, no model animal that could be used to directly analyze the morphogenetic mechanism against gravity was available, and the mechanism has not yet been elucidated. In this study, we performed large-scale screening of medaka (Oryzias latipes) mutants (Kondoh Differentiation Signaling Project, Exploratory Research for Advanced Technology [ERATO]) and identified a flat-bodied mutant variety. We named this mutant hirame (hir), which is another name for Japanese flatfish Paralichthys olivaceus. We observed that in the mutant, the yes-associated protein (YAP) transcriptional co-activator, which is the causative gene, exhibited resistance against gravity by adjusting cell tension, and controlled the construction and arrangement of three-dimensional (3D) tissues. Here, we introduce the mechanism underlying 3D organogenesis operational in the hir mutant. |
Decrease in Cell Tension in hir Mutants |
The different embryos of the hir mutants were flattened in various directions against the earth's gravity; however, they always collapsed in the direction of gravity. This indicated the existence of an abnormality in the mechanism underlying the morphogenesis of organisms against gravity, as predicted by D'Arcy Thompson. The dynamic analysis of hir mutants using the pipette aspiration method revealed decreased tissue tension in the neural tube and decreased actomyosin phosphorylation. Therefore, YAP was considered essential for generating cell tension to resist gravity.
Neural tube flattening was also attributed to the lack of cell accumulation in hir mutants in the time-lapse analysis of single-cell gene expression in live embryos (Fig. 1). Positional cloning revealed that the causative gene in hir mutation coded the YAP transcriptional co-activator. In vertebrates, YAP and the transcriptional co-activator with PDZ-binding motif (TAZ), which is a paralog of YAP, are known as effector molecules in the Hippo signaling pathway; they regulate organ sizes by inducing the expression of cell proliferation-related genes in the nucleus. Unlike the case in other model animals, TAZ is mainly responsible for regulation of cell proliferation in medaka. Therefore, a phenotype of YAP other than that attributed to cell proliferation could be identified. |
|
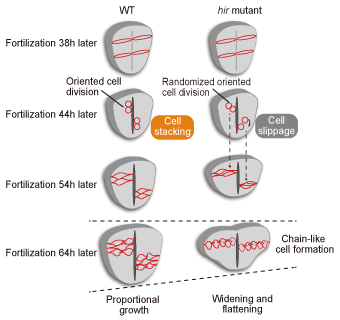
Fig. 1. Neural tube formation in wild-type medaka and hir mutants
In hir mutants, the cell division axes of neuroepithelial cells become unstable, accompanied by a decrease in tissue tension, and cannot be normally laminated due to a shift in the positional relationship between the daughter cells; consequently, the neural tube flattens.
|
Mechanism underlying Abnormal Tissue Arrangement in hir Mutants |
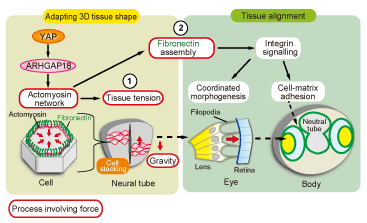
The eye develops by the invagination of the optic lens into the retinal neuroepithelium. In hir mutants, after normal differentiation, the lens segregates from the retina without invaginating into the retinal neuroepithelium. In the co-operatively morphogenetic process of lens and optic cup formation during eye development, filopodia formation between the lens and the optic cup plays an important role. However, this filopodia formation was hardly recognized in hir mutants. In addition to actomyosin, fibronectin (FN)-integrin signaling is involved in filopodia formation between the lens and the optic cup.
The presence of polymerized FN fibers between the lens and the retina was confirmed in wild-type embryos while abnormal integrin signaling, rather than normal polymerization of FN fibers, was recognized in hir mutants. During inhibition of fibronectin polymerization in normal embryos, although the optic lens segregated from the optic cup, the tissues did not flatten. Therefore, YAP was considered to induce the polymerization of fibronectin and activation of integrin signaling by controlling the activity of actomyosin, and be involved in the normal tissue arrangement of the lens and the retina. |
YAP Controls Tissue Tension and Polymerization of Fibronectin via RhoGAP |
To verify that a new function of YAP found in medaka is preserved in human cells, we could reproduce the decrease in the actomyosin activity and a phenotype of hir mutants with abnormal polymerization of fibronectin in the 3D spheroid culture system of YAP-knockdown human retinal pigment epithelium-derived hTERT-RPE1 cells. Moreover, gene expression analysis using microarrays of YAP-knockdown spheroids identified the ARHGAP18 (GTPase-activating protein 18) gene from 40 genes that showed decreased expression. The detection of an abnormality similar to that observed in YAP-knockdown spheroids in ARHGAP18-knockdown spheroids showed that YAP controls the activation of actomyosin networks and plays an essential role in controlling the tissue tension and polymerization of fibronectin via ARHGAP18 (Fig. 2).
|
 |
|
Fig. 2. Control models for 3D tissue morphology and tissue arrangement by YAP
YAP is considered to control the activation of actomyosin networks via RhoGAP (ARHGAP18). In addition, it 1) constructs a 3D morphology of tissues such as the neural tube by controlling cell tension and 2) regulates the 3D arrangement of tissues such as the lens and the retina through integrin signaling by inducing polymerization of fibronectin.
|
In summary, this study elucidated the mechanism of gravitational resistance in the whole-body morphogenesis of a model animal. This mechanism, which has been preserved in a variety of animals including fish and humans, was elucidated based on the in vivo analysis of cell tension, molecular and cellular behavior, and extracellular matrix formation, the accurate reproduction of which is difficult in vitro. The results of the present study indicate that medaka is an excellent model organism to elucidate the mechanism underlying 3D organogenesis.
Finally, I would like to thank Visiting Professor Hisato Kondoh (Kyoto Sangyo University), Professor Makoto Furutani-Seiki (The University of Bath, United Kingdom) and other collaborators for giving me an opportunity to participate in this project. I am also grateful to Associate Professor Kiyoshi Naruse (National Institute for Basic Biology) for diligently managing medaka bioresources.
|
| |
Reference |
| |
Porazinski, S et al., YAP is essential for tissue tension to ensure vertebrate 3D body shape. Nature 521,217-221 (2015) |
|