|
 |
■ Bioresources information is available at the following URLs
|
 |
|
Research and Bioresources 〈NO.10〉 Neurological Research in Ciona intestinalis by Using Kaede Fluorescent Proteins
Takeo Horie1・Yasunori Sasakura1, 2
Shimoda Marine Research Center, University of Tsukuba1, Associate Professor2
|
Ciona intestinalis as a Model Organism
|
Sea squirts, including Ciona intestinalis (Photo 1), belong to the phylum Chordata, and their larvae exhibit a typical tadpole form (Photo 1A) with a notochord and dorsal central nervous system, which are body plan*1 characteristics similar to those of vertebrates. Embryogenesis of most sea squirts is a simple process: early embryos and larvae consist of a countable number of cells and their cell lineages*2 have been elucidated. In addition, sea squirts produce so-called mosaic eggs, which indicates that each region of the fertilized egg and its corresponding body region in the adult are defined during early embryogenesis. Because of these characteristics, the sea squirt is an attractive model organism for embryogenesis research.
|
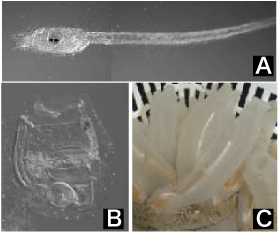 |
Photo 1: Ciona intestinalis, a sea squirt species
(A) Larva of Ciona intestinalis, which exhibits a tadpole form and swims actively.
(B) Ciona intestinalis immediately after morphogenesis, losing their tails and becoming sessile.
(C) Adult Ciona intestinalis |
|
Among sea squirts, the species Ciona intestinalis is suitable as a research resource for elucidating gene functions. The genome of Ciona intestinalis was sequenced in 20021), and the haploid genome size was estimated to be ca. 160 Mbp, with 15,852 genes. This indicates that Ciona intestinalis harbors an extremely compact genome. Moreover, the gene functions of Ciona intestinalis have been intensively studied by introducing extraneous DNAs or RNAs into eggs via microinjection. In particular, the microinjection of morpholino oligos*3, which inhibit the translation of genes, can be used to induce inhibition of gene functions. Furthermore, exogenous DNAs can be transfected into hundreds of eggs or early embryos by an electroporation method. Thus, because information about its gene sequence is available and various experimental techniques can be used to analyze its gene functions, Ciona intestinalis is considered a model organism of the phylum Chordata.
|
|
Construction of Transgenic Strains of Ciona intestinalis
Another interesting property of Ciona intestinalis is that its generation time is relatively short, at 2 to 3 months. Taking advantage of this short generation time, we have been consolidating fundamental genetic engineering technologies involving Ciona intestinalis. First, an indoor closed-breeding system was constructed. This method enabled the breeding of Ciona intestinalis and its genetically modified strains in laboratories or closed environments to obtain offspring for genetic experiments. Second, we undertook the development of transgenic strains*4. We elucidated the transposition activity of Minos, a transposon*5 belonging to the Tc1/mariner superfamily, in Ciona intestinalis2) and successfully constructed transgenic strains of Ciona intestinalis harboring Minos in their genomes (Fig. 1). |
 |
Fig. 1: Transformation of Ciona intestinalis by Minos transposon
Minos vector containing a reporter gene*6 is transfected into eggs of Ciona intestinalis with transferase mRNA. Some of the transfected individuals pass on the inserted Minos to the next generation. |
|
If the Minos transposon vector and transferase*7 mRNA are transfected into Ciona intestinalis during early embryogenesis, approximately 30% of the individuals pass on the inserted Minos to subsequent generations. This implies that the inserted Minos is inheritable in Ciona intestinalis. In recent years a strain expressing the transferase in its germ cells was constructed, and this facilitated the establishment of a new method: By crossing this strain with the Minos-inserted strain, strains harboring novel insertions can be established3) since the Minos translocates to other regions in the genome of the germ cells by the activity of the transferase. In addition, we successfully conducted enhancer trap*8,4) which detects the activity of an enhancer, a transcription regulatory region in genome, and constructed insertion mutants by inserting Minos and knocking out genes5). Many of transgenic strains constructed by using Minos have been deposited to the National BioResource Project (NBRP) and are prepared for distribution.
| *1 |
Blueprint of a biological body. |
| *2 |
Road map of the differentiation of each cell from a fertilized egg to an adult. |
| *3 |
Morpholino oligonucleotide, which is an artificial nucleic acid analog. In genetics research, morpholino oligos harboring a nucleotide sequence that is complementary to a target sequence near the translation initiation site of mRNA are primarily constructed and, once injected into cells, these oligos inhibit translation by binding to the mRNA. |
| *4 |
Strains harboring exogenous DNAs that are not intrinsically contained in the organism's genome. |
| *5 |
DNAs with a property of translocating in an organism's genome DNA, characteristically having terminal repeated sequences. |
| *6 |
Generic term for genes that are used to easily detect a certain biological phenomenon. |
| *7 |
Enzyme that is required for the translocation of transposons. |
| *8 |
If a reporter gene is inserted adjacent to a region of genomic DNA with enhancer activity, expression of the reporter gene will be regulated and changed by the enhancer. Thus, enhancer trap is a method to detect the enhancer in the genome by observing changes in the reporter gene expression. |
|
|
Ciona intestinalis Strains Expressing Kaede
Kaede is a fluorescent protein6) isolated from corals and is characterized by its ability to change color from green to red on exposure to ultraviolet (UV) light (photoconversion activity). Green fluorescent proteins derived from Aequorea coerulescens can also illuminate cells; however, Kaede fluorescent proteins, by virtue of their photoconversion activity, can be used for precisely marking cells in a location- and time-specific manner, thereby enabling the determination of subsequent changes in the cells.
Ciona intestinalis harboring Kaede expressed in the entire nervous system or specific neurons, such as cholinergic, GABAergic, glycinergic, glutamatergic, and dopaminergic neurons, were developed (Photo 2) and deposited at NBRP. Larvae of sea squirts exhibit a simple nervous system consisting of ca. 100 neurons; therefore, the strains expressing Kaede in these neurons are promising as powerful tools in research on the entire neural network. |
 |
Photo 2: Transgenic strains expressing Kaede in the nervous system of a larva
(A) Larval strain expressing Kaede in its entire nervous system
(B) Larval strain expressing Kaede specifically in cholinergic neurons
|
|
|
Tracing neurons during the morphogenetic process by using the property of photoconversion of Kaede
We present an example of research based on the aforementioned Kaede strain. Sea squirts appear to be of the tadpole form and actively swim in their larval stage but become sessile after morphogenesis (Photo 1). On the basis of these properties, the central nervous system of sea squirts is believed to change considerably during morphogenesis, along with dramatic lifestyle changes. A previously accepted theory states that during morphogenesis, the central nervous system of larval sea squirts is completely lost and that of adult sea squirts is newly formed. However, no experiment has hitherto precisely traced the morphogenetic process of neurons of larval sea squirts and clarified whether or not the larval neurons disappear after morphogenesis.
We sought to clarify these issues by using the aforementioned Kaede strain. Specifically, we first labeled the neurons of the central nervous system of larvae by red fluorescence of Kaede (Photo 3A). We then investigated whether the cells emitting the red fluorescence remain or disappear after morphogenesis and found that most larval cells originating from the central nervous system were retained in the adult even after morphogenesis (Photo 3B and 3C). Subsequently, we traced the cells in the central nervous system to identify the types of cells that remain after morphogenesis and found that the majority of larval neurons disappear and the cells remaining after morphogenesis primarily include ependymal cells, which are larval glia cells. (Ependymal cells are the only glia cells that have been reported in the central nervous system of sea squirts.) |
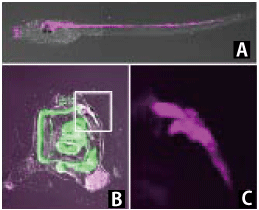 |
Photo 3: Tracing experiment of larval neurons during the morphogenesis of sea squirts by using Kaede
(A) Larval neurons labeled by red fluorescence using the photoconversion of Kaede
(B) Picture confirming that neurons of the larva labeled with red fluorescence shown in (A) were retained in the central nervous system after morphogenesis
(C) Enlarged picture showing the boxed region in (B)
|
|
Moreover, a part of the ependymal cells was found to differentiate into neurons during morphogenesis and compensate for neurons in adults. In other words, during the morphogenesis of sea squirts, larval glia cells function as neural stem cells and thus give rise to adult neurons7). In this manner, Kaede strains can be a powerful tool for tracing experiments of cells. Kaede strains as well as transgenic strains of specific tissues labeled with fluorescent proteins are also extremely useful to clarify the mechanism of embryogenesis. Only NBRP resources in Japan maintain such sea squirt strains in the world. We would like to elucidate the mechanism of embryogenesis in sea squirts from a creative point of view, by using Japanese-born resources. |
References
1) Dehal et al., 2002. Science 298:2157-2167.
2) Sasakura et al., 2003. PNAS 100:7726-7730.
3) Sasakura et al., 2008. Dev. Dyn. 237:39-50.
4) Awazu et al., 2004. Dev. Biol. 275:459-472.
5) Sasakura et al., 2005. PNAS 102:15134-15139.
6) Ando et al., 2002. PNAS 99:12651-12656.
7) Horie et al., 2011. Nature 469:525-528. |
|
Collective Manipulations of Images
|

Several tools, such as Microsoft Paint and Photoshop, are currently available for manipulating images. Although these tools help change the size and format of images or write texts on images, the processing of a large number of images is time-consuming. In this issue, we introduce "ImageMagick," which is useful in such circumstances.
ImageMagick (http://www.imagemagick.org/) is an image-processing tool equipped with several functions, such as changing the size and format of, writing texts on, and superposing images. ImageMagick entails the use of command lines but is suitable for effective processing of a volume of images.
|
|
 Installation of ImageMagick Installation of ImageMagick
Access the URL above and download ImageMagick. Among the Linux, Windows, and MacOS versions available, the Windows binary package version is used in this example (Fig. 1). Installation will begin by executing the downloaded .exe file. Please follow the instructions of the installer.
Fig. 1
|
|
 |
|
| |
 Processing by a command line Processing by a command line
Run command prompt after the installation.
(Start Menu → Accessories → Command Prompt)
Go to the directory containing the images to be processed in order to run the commands. Many commands are available; the commands include "convert" (single image conversion), "mogrify" (multiple image conversion), and "combine" (superposition) and options include "resize" (size change), "annotate" (text writing), and "format" (format change).
※Reference website:http://www.imagemagick.org/Usage
The following image processing steps are demonstrated in this example: All the jpeg images (*.jpg) should be output as png images with the size of 320 px (height) × 240 px (width) and with the text message, "National BioResource Project", written on the coordinate of 50 px × 50 px with the font size of 18 px.

※The command is one line although it is written in multiple lines here due to the limitation of space. "Δ” indicates half-sized space.

Collective image processing can be performed by a single command, as demonstrated above. In addition, ImageMagick is also accompanied by an image-processing window. Run ImageMagickDisplay by clicking on "imdisplay" icon on the desktop or "ImageMagickDisplay" in the start menu. However, ImageMagickDisplay is not as multifunctional as the command version. In addition, ImageMagick can be called up from various programming languages. (In Perl, "Image : : Magick" should be mounted for this purpose.)
ImageMagick is highly recommended when many images are to be processed by a single script.
|
|
|
Please note that "magick" and "mogrify" are not typos.
(Manabu Hattori, Center for Genetic Resource Information) |
|
|