|
 |
■ Bioresources information is available at the following URLs
|
 |
 |
■ Announcements
|
 |
|
Research and Bioresources 〈No.9〉 Development of Transgenic Medaka (Oryzias latipes) Strains with Inducible Gene Expressions in a Stage- and Tissue-specific Manner
Kayo Kobayashi1・Shuhei Nakamura1・Minoru Tanaka1,2,3
Laboratory of Molecular Genetics for Reproduction, National Institute for Basic Biology1, Associate Professor2, Department of Basic Biology, the Graduate University for Advanced Studies3
 Medaka as a Model Research Organism |
Medaka was established as a model research organism in Japan, given its numerous advantages: medaka can be maintained easily and inexpensively, and its eggs are transparent and thus suitable for developmental research. Moreover, inbred strains of medaka have been developed, and their full genome has been sequenced. Taken together, these advantages promise the elucidation of various biological phenomena that have been difficult to investigate using other organisms as biological models.
However, genetic engineering technology that artificially manipulates genes, as in the case with mice, has not been established in medaka; therefore, the development of knockout medaka models harboring dysfunctional genes is difficult. Hence, instead of directly manipulating genes, a method that inhibits gene functions by using morpholino antisense oligonucleotides*1(the knockdown method) has been employed in medaka. This method, however, can only be used in early development and affects all the cells, and thus, it is inappropriate for the research in the late developmental stages or specific cells in adult fish. Despite the wide array of biological phenomena that occur in the late stages of development that remain to be elucidated, such as problems in physiology, endocrinology, and regeneration, the effects of gene functions and genetic mechanisms have remained mostly unresolved. Hence, in order to manipulate gene function in late developmental stages or in adulthood, we attempted to develop transgenic medaka strains (hsp-cre、gapdhp-loxP) in which gene expression can be induced at arbitrary stages and in a variety of tissues, by a combination of cre/loxP system*2 and heat induction.
*1 Morpholino antisense oligonucleotides: Nucleic acid analogs harboring 4 bases similar to RNA bases, but with different chemical structures, in the backbone moiety. Once incorporated into the cells, the molecules bind to mRNA containing complementary sequences and inhibit their translation (Biology Dictionary/Tokyo Kagaku Dojin).
*2 cre/loxP system: cre is a recombinant enzyme (recombinase) that recognizes a specific DNA sequence (loxP) and recombines the sequences. If 2 loxP are aligned in the same direction, cre excises the region flanked by the loxP. This mechanism can be used to manipulate gene expression by determining the regulatory regions of cre and loxP.
|
 Development of transgenic medaka strains (cre-loxP) |
A simplified diagram of the cre/loxP system currently in use is shown in Fig. 1. In order to examine gene functions in organisms, it is necessary that the site and the time (stage) at which the genes are expressed be regulated. For example, regulatory region A, which shows activity in tissue A, will be situated upstream of the red-fluorescent protein (DsRed) gene, which is expressed in a specific tissue. As a result, the DsRed gene expression is activated in tissue A, which will show red fluorescence. Next, the induction of cre recombinase expression at an arbitrary stage was attempted in order to regulate the timing of the fluorescent expression. If these techniques are combined, the region containing the DsRed gene flanked by 2 loxP will be excised at the specific time at which the cre recombinase expression is induced; then, the enhanced green fluorescent protein (EGFP) gene situated downstream of the DsRed gene will be expressed. This new expression derived from the EGFP gene can be discriminated by its green fluorescence.。
|
| 
Fig. 1: Schematic of cre/loxP
Without the cre recombinase, DsRed expression is activated by regulatory region A. After heat induction, the sequence flanked by the loxP will be excised via cre recombinase, and EGFP expression will be initiated. |
Here, by replacing the EGFP gene and regulatory site A with gene X and regulatory region B of the researcher’s choice, gene X can be expressed in tissue B at an arbitrary time, and the function of X can then be examined.
We developed a transgenic medaka (hsp-cre strain) in which cre recombinase can be expressed at a controlled time by introducing heat shock elements (HSEs) into the regulatory region of the cre gene. In order to examine whether the cre gene of the developed hsp-cre strain can function in any tissue, the regulatory region of gapdh, which is expected to be expressed in many tissues, was employed as the loxP site (gapdhp-loxP strain). |
 Heat induction in a medaka embryo |
|
Two types of medaka strains, hsp-cre and gapdhp-loxP, were independently developed and crossed; the medaka harboring both cre and loxP were obtained to investigate the effect of heat induction.
An embryo at stages 32–33, the segment formation stage, was heated at 39°C for 2 h and left to rest at room temperature for 7 days; then, EGFP expression was examined. As a result, the induction of the EGFP expression was confirmed in a part of the cells in the heart, liver, scale, colon, muscle, and archinephron (Fig. 2). This result indicates that heat induction of the expression of arbitrary genes is feasible using hsp-cre strains in these tissues. |
| 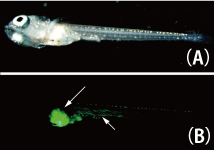
Fig. 2: EGFP Induced in Medaka Embryos.
(A) Bright-field image of medaka and (B) induced EGFP observed in muscle and bowel tissues, as indicated by arrows. |
 Heat induction in adult medakas |
Subsequently, heat induction was performed in adult medakas. Two methods, hot bath and thermal stimulator, were employed for this experiment. Although the hot bath method is more convenient and thus more frequently used, there exists the undesirable possibility that HSEs are activated in the whole body. In the hot bath method, the temperature of tank water was increased from 30°C to 39°C in 3°C increments every 30 min, and then maintained at 39°C for 2 h. Then, the medaka were raised for 14-16 days at room temperature, and the induction of EGFP expression was observed in part of the ovary cells. |
In contrast, in the thermal stimulator method, the tip of a long, thin probe (50-mm long and 1.5-mm thick) connected to a thermal stimulator (BTC-201; Unique Medical) was inserted into the abdominal cavity; thus, heat was induced locally (Fig. 3). We applied local heat at 40–42°C for 10 min, then raised the medaka for 14-16 days at room temperature. As a result, EGFP expression was observed in the vicinity of the ovary where the probe was applied. It is expected that this method will enable heat induction in a small region in a non-invasive manner. |
| 
Fig. 3: Heat Induction via Probe in an Adult Medaka.
Heat induction by inserting the tip of a probe into the abdominal cavity of an adult medaka. |
 Application example |
Long an important question, there has been no way to examine whether germ stem cells in the ovaries of adult vertebrates can continuously produce eggs. Previously, in our laboratory, Aoki et al. identified that the nanos2 gene was expressed in ooblasts of medaka ovaries1). Nakamura et al. then used the medaka we have developed in order to trace the progeny of the nano2-expressing cells; they used a strain containing the regulatory region of nano2 as the loxP site and hybridized the strain with hsp-cre strains (Fig. 4). |
| First, the sequence flanked by loxP was excised via heat treatment, and green fluorescence was observed in nano2-expressing cells. In addition, when the green fluorescence was traced, the eggs continuously emitted green fluorescence for 3 months; thus, Nakamura et al. successfully showed for the first time that vertebrate ovaries contain nano2-expressing germ stem cells2).
In this manner, the medaka strains we developed can be used to elucidate previously-unresolvable gene functions and biological phenomena at later developmental stages or in adult fish.
References
1) Aoki et al., 2009. Zool. Sci 26:112-118.
2) Nakamura et al., 2010. Science 328:1561-1563.
|
|

Fig. 4: Clone Analysis of nano2-Expressing Cells.
(A) Schematic of clone analysis.
(B) EGFP expression was observed only in nano2-expressing cells immediately after induction (left), but was later observed in all the germ cells (right). |
 Acknowledgments
HSE vectors were provided by Dr. Czerny, cre and loxP vectors by Dr. Higashijima at the National Institute for Physiological Sciences, and fosmids by the National BioResource Project (NBRP) Medaka. |
|
COP 10 〈Three-part Series〉
Convention on Biological Diversity and the Circumstances
Surrounding Genetic Resources (1)
"Historical Success and the Adoption of the Nagoya Protocol"
Mutsuaki Suzuki, Director, Intellectual Property Unit,National Institute of Genetics |
 |

Introduction
"Access to and Benefit-Sharing," "Preservation," and "Continuous Use" of genetic resources are 3 critical themes in the Convention on Biological Diversity. The 10th Conference of the Parties to the Convention on Biological Diversity (COP10) was organized in Nagoya in October 2010, and the Nagoya Treaty, which exhibits legal binding force regarding the access to and benefit-sharing of genetic resources, was adopted. In this edition of the newsletter, issues introduced by COP10 and the Nagoya Protocol are explained, and related circumstances surrounding genetic resources will be introduced.
|
|

Working Group |

Toward the Adoption of the Nagoya Protocol
A provisional protocol was laid out in March 2010 in Cali, Colombia, and many ideas were proposed at three preliminary conferences; however, no advancement towards agreement was achieved. In COP10, despite the daily discussions that continued until late night, there was little actual advancement. The disagreement between developing and advanced countries, particularly regarding derivatives, retroactive application, and monitoring, remained unresolved and most affiliates thought that the protocol would not be adopted.
The consensus document was not completed in the working group, and unconventionally, a chairperson’s proposal was put forth on the final day. The proposal was a compromise resolution containing some propositions in favor of advanced countries, with additional financial supports proposed simultaneously. What happened next? All the participants were nervous about the consequences; however, the proposal was adopted at the top-level meeting and then at the following general meeting, and the participants were grateful for the historic consensus. It was 3 o’clock in the morning when the meeting was finally concluded.
|
|

Delighted participants after the final plenary session
|

Showdown over the problems?
Ultimately, it was stipulated that derivatives are individually-addressed contracts; and it became mandatory to set one or more checkpoints to ensure that the use of genetic resources adheres to legal standards. In addition, retroactive clauses were not included; instead, multilateral profit-sharing mechanisms were considered. Beginning in February 2011, signed agreements from each country will be sought and placed into effect. In the future, inter-governmental committee meetings will be organized to negotiate specific regulatory needs. Specifically, the development of domestic rules and the installation of regulatory checkpoints are also expected in Japan, with particulars to be introduced in next month’s issue. |
|

A work of fine art decorated with folded origami
|
| ● The official website of the COP10 support committee: http://www.cop10.jp/aichi-nagoya/english/index.html |
| |
|