Memorial Issue, Wheat Information Service No.100
Abiotic stress signal pathways associated with development of freezing tolerance after cold acclimation in common wheat
S. Takumi* and C. Nakamura
Laboratory of Plant Genetics, Department of Biological and Environmental Sciences, Faculty of Agriculture, Kobe University, Rokkodai-cho 1-1, Nada-ku, Kobe 657-8501, Japan
*: Corresponding author: Shigeo Takumi
E-mail address: takumi@kobe-u.ac.jp
Key words: ABA, CBF regulon, cold acclimation, Cor /Lea genes, freezing tolerance, Fr-1, Triticum aestivum, Vrn-1
Introduction: Abiotic stress signal pathways in Arabidopsis
Cold/freezing temperatures represent one of the significant abiotic stresses limiting geographical distribution of plants and reducing crop quality and productivity. Cold/freezing tolerance, an important trait for adaptation of over-wintering temperate species to low temperature (LT), is acquired through cold acclimation that is triggered in response to low but non-freezing temperatures for certain periods of time (Levitt 1980; Guy 1990; Thomashow 1999). In the cold-acclimation process, a large number of stress responsive genes are transcriptionally activated, and the accumulated proteins and metabolites lead to the protection of integrity of cell structures and functions from freezing damage.
Transcriptome analyses in Arabidopsis and rice have revealed that many signaling components are involved in the LT signaling pathways (Seki et al. 2002; Rabbani et al. 2003). Expression of a series of Cor (cold-responsive)/Lea (late-embryogenesis-abundant) genes as the LT signaling components and accumulation of their COR/LEA proteins contribute to promoting the development of freezing tolerance (Thomashow 1999). In Arabidopsis, a functional cis -acting element, i.e. the CCGAC core motif known as a CRT (C-repeat)/DRE (dehydration-responsive element) sequence, was proven to play a pivotal role in the promoter function of COR15A /RD29A genes (Yamaguchi-Shinozaki and Shinozaki 1994; Baker et al. 1994). Both ABA-dependent and -independent pathways regulate the expression of Arabidopsis Cor/Lea genes (Shinozaki and Yamaguchi-Shinozaki 2000). A family of transcription factors called CRT-binding factors (CBFS) or DRE-binding proteins (DREBs) is involved in the ABA-independent pathway and regulates the Cor /Lea gene expression through binding to the CRT/DRE cis elements. These transcription factors contain a DNA binding domain found in the ethylene-responsive element binding protein/ APETALA2 (EREBP/AP2) family (Stockinger et al. 1997; Liu et al. 1998). In Arabidopsis, three tandemly-duplicated CBF/DREB1 genes, CBF1 /DREB1B, CBF2 /DREB1C and CBF3 / DREB1A, are induced by LT. The CBF /DREB1 transcript levels reach a maximum at about 3 h LT treatment and then decline significantly, and the transcripts remain at a low level over the course of the 3-week LT treatment (Zarka et al. 2003). The rapid induction of CBF3 /DREB1A is controlled by the constitutively expressed ICE1 (inducer of CBF expression 1) gene (Chinnusamy et al. 2003). The ICE1 protein belongs to the MYC-like basic helix-loop-helix (bHLH) transcription factor family. Expression of CBF1 /DREB1B and CBF3 /DREB1A precedes that of CBF2 /DREB1C, and analysis of the cbf2 mutant revealed that the CBF2/DREB1C protein functions as a negative regulator of the CBF1 / DREB1B and CBF3 /DREB1A expression (Novillo et al. 2004). The CBF-mediated cold-response system in Arabidopsis appears to be conserved in both dicotyledonous and monocotyledonous plants including rape, tomato, wheat and rye (Jaglo et al. 2001; Hsieh et al. 2002).
Abscisic acid (ABA) is one of the key plant hormones responding to environmental stresses. Exogenous application of ABA induces a number of genes that respond to LT and drought stresses (Thomashow 1999). On the other hand, some genes that are induced by LT and drought do not respond to exogenous ABA treatment. Therefore, it is likely that both ABA-dependent and ABA-independent signal transduction pathways are involved in the LT and drought responses of plant cells (Thomashow 1999; Shinozaki and Yamaguchi Shinozaki 2000). Arabidopsis DREB2 transcription factor recognizes the CRT/DRE motif and belongs to the EREBP/AP2 family similar to CBF/DREB1. The DREB2 gene expression is ABA-independent and induced by drought stress but not by LT stress (Liu et al. 1998). Expression of a CBF4 gene, which was recently isolated as another member of the CBF / DREB family, is up-regulated by drought stress through the ABA-dependent pathway (Haake et al. 2002). Because many LT- and drought-inducible genes contain both CRT/ DRE and ABA-responsible element (ABRE) motifs in their promoters, these cis elements are considered to function independently. A recent study of the Arabidopsis RD29A promoter showed that the CRT/DRE functions cooperatively with ABRE as a coupling element in the ABA-responsive gene expression in response to drought stress (Narusaka et al. 2003). Such regulatory pathways in Arabidopsis provide considerably informative models to study the LT/drought signal response in monocotyledonous crop species.
In common wheat, Vrn-1 and Fr-1 are well known major quantitative trait loci (QTLs) respectively determining vernalization requirement for flowering and winter hardiness. The two loci are linked on the long-arms of homoeologous group 5 chromosomes (Galiba et al. 1995; Sutka et al. 1999). We herein summarize our recent studies of wheat cold-signaling pathways and its relation to the Fr-1 locus. The CRT/DRE, ABRE and other cold-responsive motifs have been identified in the 5' upstream regions of many Cor /Lea genes in common wheat. Wcbf2 genes encode a putative transcription factor recognizing the CRT motif (Takumi et al. 2003b). The WCBF2 cascade is tightly associated with cold acclimation and the Fr-1 alleles, whereas expression of the cold-responsive Vrn-1 candidate gene WAP1 is apparently not related to expression of the Wcbf2 gene (Kobayashi et al. 2005). Expression of other cold-responsive transcription factor genes, Wdreb2 and Wlip19, are weakly controlled by Fr-1, while Wabi5 transcription factor gene involved in the ABA-dependent pathway appears to act independently of Fr-1 (Kobayashi et al. 2004b). Mutant analyses suggest that ABA sensitivity is not associated with cold acclimation, while ABA is involved in determination of the basal level of freezing tolerance in wheat (our unpublished data). Thus, the CBF-mediated Cor /Lea gene expression plays central roles in cold acclimation to develop high levels of freezing tolerance in wheat. Under this situation, the isolation of the Fr-1 locus has become an important and urgent goal for many wheat researchers working in this area.
QTLs for freezing tolerance in wheat
In Triticeae species such as common wheat (Triticum aestivum L.), barley (Hordeum vulgare L.) and rye (Secale cereale L.), a number of LT-inducible Cor /Lea and CBF /DREB -related genes have been isolated and characterized, and genetic maps of major loci and QTLs affecting cold/freezing tolerance have been constructed and compared (Cattivelli et al. 2002). The loci influencing freezing tolerance were assigned to the homoeologous group 5 chromosomes and chromosomes 4B, 4D and 7A through the studies using monosomic and chromosome substitution lines of common wheat (Law and Jenkins 1970; Sutka 1981; Roberts 1986; Galiba and Sutka 1988; Veisz and Sutka 1989). Chromosomes 5A and 5D have been implicated most frequently to carry major regulatory genes (Sutka 2001; Snape et al. 2001). A study using chromosome substitution lines revealed that the loci for frost resistance were located on the chromosomes 5A and 5D, and the locus on the chromosome 5A was originally designated as Fr1 (now Fr-A1 ) (Sutka and Snape 1989). A second freezing tolerance locus, Fr2 (now Fr-D1 ), which is homoeologous to Fr-A1, was mapped on chromosome 5DL (Snape et al. 1997). On the chromosome 5BL, another homoeologous locus Fr-B1 was mapped (Toth et al. 2003). Recently, a new locus, Fr-A2, has been identified and mapped on a distal region of the long arm of chromosome 5Am in diploid wheat T. monococcum (Vagujfalvi et al. 2003).
In Triticeae, the homoeologous group 5 chromosomes exert a major effect on both freezing tolerance and vernalization requirement. Vernalization requirement, which necessitates certain periods of exposure of winter-type plants to LT for ensuring transition from the vegetative phase to the reproductive phase, is another critical trait for cold adaptation and seems to be tightly associated with cold/freezing tolerance. The Vrn-A1 and Fr-A1 loci, which are intimately linked on the chromosome 5AL in common wheat, are the major led controlling the vernalization requirement and freezing tolerance, respectively (Galiba et al. 1995; Sutka et al. 1999). The Fr-A1 locus was formerly considered to be completely linked to the Vrn-A1 locus on chromosome 5AL in common wheat, and it was suggested that the freezing response was a pleiotropic action of the Vrn-A1 locus since the spring allele was associated with sensitivity to freezing damage (Sutka and Snape 1989; Brule-Babel and Fowler 1988). Mapping of the chromosome region containing Vrn-H1 and Fr-H1 on chromosome 5H was achieved in barley (Pan et al. 1994), followed by mapping of Vrn-A1 and Fr-A1 on chromosome 5AL in common wheat (Galiba et al. 1995). According to the latter study, the genetic distance between the Vrn-A1 and Fr-A1 loci was estimated to be ca. 2 cM, suggesting that the vernalization requirement and frost resistance are affected by two separate genetic systems (Cattivelli et al. 2002, for review). Homozygous deletion lines for chromosome 5AL were tested for flowering time without vernalization and for freezing tolerance after cold acclimation (Sutka et al. 1999). Analysis of flowering time revealed significant differences between the lines possessing and lacking the Vrn-A1 locus. According to the freezing tolerance test in the deletion lines, lines possessing the Fr-A1 locus showed 13% higher survival rate than the lines lacking it (Sutka et al. 1999). The Vrn-A1 and Fr-A1 loci were physically mapped to the close but separate regions on chromosome 5AL. These results thus supported the previous hypothesis that vernalization and freezing tolerance were controlled by different loci. Many spring-type wheat accessions, which allow the phase transition without vernalization, carry dominant Vrn-A1 alleles. In contrast, winter-type wheat accessions, which require vernalization to promote floral development, carry recessive vrn-A1 alleles. Winter-type wheat should possess at least one dominant 'winter-type' Fr-A1 allele that guarantees winter survival but such allele is unnecessary for spring-type wheat (Thomashow 1999). In fact, bioassay of freezing tolerance using the near-isogenic lines (NILs) carrying the Vrn-A1 /Fr-A1 intervals from different varieties showed that lines carrying the 'winter-type' vrn-A1 /Fr-A1 were about 4°C more tolerant than lines carrying the 'spring-type' Vrn-A1 /Fr-A1 locus (Storile et al. 1998).
The VRN1 (Vrn-Am1) locus of T. monococcum was found to be completely linked to MADS box genes, AP1 (APETALA1) and AGLG1 (AGAMOUS -like gene), but their expression patterns suggested that AP1 was a better candidate gene for VRN1 than AGLG1 (Yan et al. 2003). VRN1 of T. monococcum was considered to be an ortholog of WAP1 (wheat AP1) in common wheat (Murai et al. 2003). Expression analysis of WAP1 showed that the accumulation of transcripts was induced by vernalization in winter-type wheat and NILs with recessive vrn-1 alleles, while constitutive accumulation of WAP1 transcript was detected in the spring-type wheat and NILs carrying the dominant Vrn-1 allele (Trevaskis et al. 2003; Murai et al. 2003; Danyluk et al. 2003). WAP1 was assigned to the region near the Vrn-A1 and Vrn-D1 loci by the deletion mapping, and was shown to function as a key gene promoting the transition from the vegetative phase to the reproductive phase in cereals (Murai et al. 2003; Danyluk et al. 2003). Large deletions in the first intron of the WAP1 loci are related with the dominant alleles of Vrn-1 (Fu et al. 2005). These results strongly suggest that WAP1 is a candidate gene for Vrn-1 in common wheat.
Wheat LT-responsive Cor /Lea genes
A developmental time-course of freezing tolerance was monitored under LT conditions in two wheat cultivars, 'Mironovskaya 808'(M808, a winter type) and 'Chinese Spring' (CS, a spring type). M808 was bred in Mironovskaya Institute, Ukraine, and reported to be the hardiest winter cultivar among tetraploid and hexaploid wheats tested for freezing tolerance (Veisz and Sutka 1990). In the simple one-point bioassay (Ohno et al. 2001), 7-day-old seedlings grown under the standard temperature at 25°C with the standard 16 h light/8 h dark photoperiod were transferred to 4°C with the same photoperiod. The LT-treated seedlings were then transferred and frozen at -15°C for 6 h in the darkness. The frozen seedlings were thawed overnight (approximately 15 h) at 4°C and returned back to the standard temperature condition for re-growth. In this freeze-thaw assay, the above-ground tissues of all the tested seedlings, irrespective of the cultivars, became wilted and withered within a day of transfer back to the standard temperature condition. LT-treated seedlings, however, showed recovery from the freezing damage and they developed new shoots from the surviving meristems within 1-2 days (Fig. 1A). The bioassay showed that the LT treatment significantly increased the level of freezing tolerance in both cultivars (Fig. 1B), and thus suggested that the LT-treated seedlings were cold-acclimated within the period of exposure to the LT condition. The cultivar difference in the freezing tolerance became significant after 3 days of cold acclimation and their levels increased until 5-7 days (Fig. 1C). The winter cultivar, M808, developed much higher levels of freezing tolerance than the spring cultivar, CS, during this early stage of cold acclimation. Moreover, the bioassay showed that the cold acclimation for 2-3 weeks gave high levels of freezing tolerance in both M808 and CS and that the long-term LT treatment dramatically increased the level of freezing tolerance only in M808 (Kume et al. 2005; Figs. 1B and 1C). M808 developed perfect freezing tolerance after 5-week cold acclimation. By contrast, in CS only about 20% seedlings survived after 5-week acclimation and all seedlings were completely killed by the freezing treatment after more than 6-week cold acclimation. The short-term and long-term cold acclimation thus demonstrated the clear difference in the ability of freezing tolerance between the two wheat cultivars.
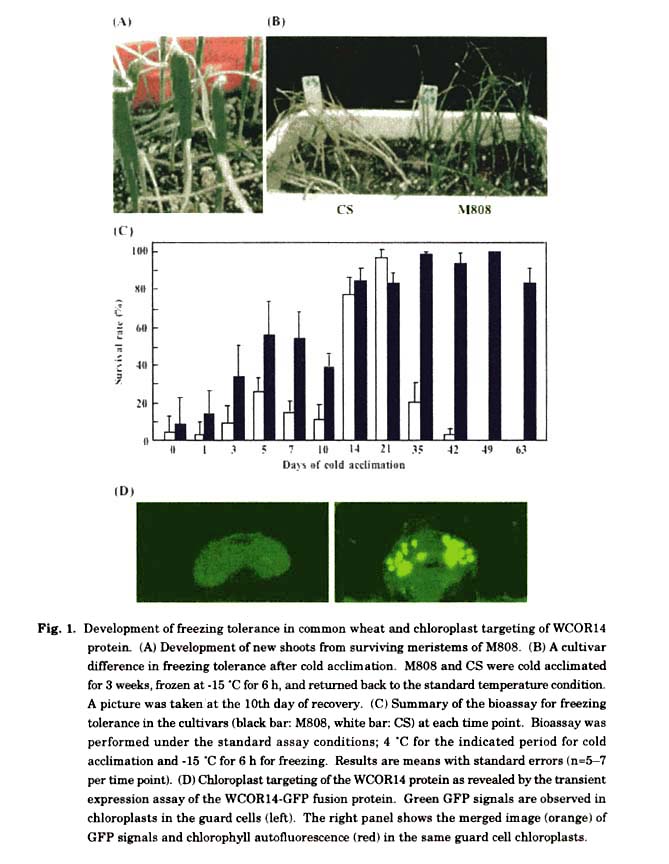
During the cold acclimation process, plants exhibit dramatic alterations in their gene expression profiles, which are characterized by the induction of a battery of cold responsive genes (Guy et al. 1985). In the last decade, a number of cold-responsive genes have been identified and characterized that are induced or activated during cold acclimation in wheat, barley and rye. These genes are independently classified as Lea, Dhn (dehydrin), Rab (response to abscisic acid), Lt (LT responsive), Cor and others. Since a majority of these genes belong to the Lea family that commonly encode highly hydrophilic proteins, they can be collectively called a Cor /Lea gene family according to the designation by Thomashow (1999). Some of the wheat Cor /Lea genes encode chloroplast-targeting proteins such as WCOR14 and WCOR15 (Tsvetanov et al. 2000; Takumi et al. 2003a). The chloroplast targeting was proven by the bombardment-mediated transient expression of chimeric CaMV35S promoter:: Wcor15-GFP and CaMV35S promoter::Wcorl4-GFP constructs in the guard cell chloroplasts of excised spiderwort leaves (Takumi et al. 2003a; Fig. 1D). Interestingly, WCOR14 and WCOR15 share an identical chloroplast-targeting signal, suggesting that Wcor14 and Wcor15 are paralogues possibly generated by duplication in the ancestral diploid genomes. Variations of copy number of the Cor /Lea genes were observed among different wheat genomes at least in Wcor15 and Wdhn13 (Takumi et al. 2003a; our unpublished data). Diversity and molecular evolution of the Cor /Lea genes should be further studied in diploid wheat genomes.
Expression of Wcor14 and Wcor15 are specifically induced by LT similar to that of Wlt10and Wcs120 (Houde et al. 1992; Tsvetanov et al. 2000; Ohno et al. 2001; Takumi et al. 2003a). Other Cor /Lea genes including Wrab17, Wrab18 and Wrab19 are responsive to LT, drought and exogenous ABA treatment (Tsuda et al. 2000; Kobayashi et al. 2004a). Both ABA-dependent and ABA-independent pathways are associated with regulation of the Cor /Lea gene expression. Moreover, transcription of the majority of Cor /Lea genes was enhanced by the light and suppressed by the darkness under the LT condition. Although the Cor /Lea genes distribute on various chromosome groups in common wheat (Kobayashi et al. 2004b, for summary), most of them exhibit a quite similar expression pattern to LT (Kobayashi et al. 2004a). This observation indicates that the wheat Cor / Lea genes are under control of a limited number of regulatory genes. The Cor /Lea genes rapidly respond to LT and their transcript levels reach high plateaus within 3-5 days (Ohno et al. 2001, 2003; Takumi et al. 2003a; Kobayashi et al. 2004a). The overall gene expression profiles are correlated well with the time-dependent development and the level of freezing tolerance under LT in the two common wheat cultivars, M808 and CS. Such positive correlation has been observed between the level of Cor /Lea expression and that of freezing tolerance in other cereals (Grossi et al. 1998; Pearce et al. 1998; Baldi et al. 1999). Over-expression of one wheat COR/LEA protein WCS19 leads to increased freezing tolerance in Arabidopsis, although only in cold-acclimated leaves (NDong et al. 2002). Over-expression of Wcor15 also improves the freezing tolerance in tobacco tranensformants (Shimamura et al. 2005). The improvement by WCOR15 however occurs only under limited freezing conditions. Influence of each COR/LEA protein on cold acclimation and freezing tolerance thus might not only be small but also spatially restricted, and a set of COR/LEA proteins should cooperatively contribute to freezing tolerance. M808 develops much higher levels of freezing tolerance than CS throughout cold acclimation periods (Ohno et al. 2001; Kobayashi et al. 2004a). During the acclimation period, M808 accumulates more COR/ LEA proteins such as WDHN13, WCOR14 and WCOR15 than CS (Ohno et al. 2003; Kobayashi et al. 2004a). The protein accumulation agrees very well with that of the corresponding transcripts and the developmental time-course of freezing tolerance. Cultivar differences in the level of freezing tolerance in wheat thus likely result from different levels of transcript accumulation of Cor /Lea genes probably regulated through the same or overlapping signal pathways.
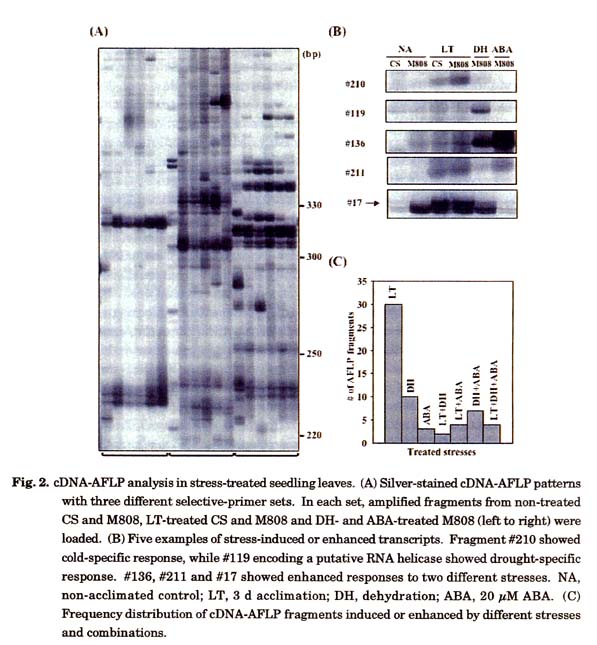
To further monitor cultivar differences in the gene expression level, expression profiles of stress-induced genes were compared by the cDNA-AFLP (amplified fragment length polymorphism) method using total RNA extracted from seedling leaves of the two cultivars grown under the LT, drought and ABA stress conditions (Fig. 2A). Two independent experiments were performed, and differentially expressed fragments (double-stranded cDNAs) that were consistently detected in the stressed leaves were scored. Some fragments that seemed to represent cultivar-dependent and/or other indefinable polymorphisms were not scored as stress-responsive. Twenty-five sets of selective primer combination generated a total of 341 cDNA fragments. Among them 60 fragments were stress-induced or at least activated by the treatment either with LT, drought or ABA (Figs. 2B and 2C). It was noted that more than a half of the transcriptionally activated sequences (19 out of 36) were LT-specific under the experimental conditions. Furthermore, the frequency of LT specific fragments was three-fold higher than that of drought-specific fragments and the combined frequency of LT-specific and LT-inducible fragments was more than 70 % of all the stress-induced fragments detected. Of the 60 stress-inducible fragments, 36 were cloned in pGEM-T vector and sequenced. Only five sequences showed detectable homology to previously reported genes. One DH-responsive fragment was identical to a cDNA sequence encoding protein disulfide isomerase (PDI) of common and emmer wheats (Ciaffi et al. 2001). PDI plays an important role in folding of plant secretary proteins and particularly in the formation of protein bodies in cereal endosperm during seed maturation. Another fragment induced by the LT treatment showed high homology to a gene for heat shock protein 16.9B of common wheat (accession number X64618). Three other LT-specific fragments (#1, #17 and #18; #17 in Fig. 2B) were predicted to encode proteins homologous to ATP-dependent RNA helicases of rice and Arabidopsis (accession numbers AP003822-16 and AC01661-29, respectively). These fragments might be derived from paralogous or homoeologous wheat mRNAs because they were highly homologous with each other. The other fragments showed no homology to known functional genes and were in many cases identical to wheat and/or barley EST clones registered in the DNA databank. Four of such EST clones were derived from cold-stressed cDNA libraries and five were from drought-and salt-stressed libraries. RT-PCR analysis using four of the selected 36 fragments confirmed that their expression was in fact LT specific (data not shown). Despite a limited scale of the cDNA-AFLP analysis, these results suggest that ABA-independent cold-specific signal pathways predominate under tested short-term stress conditions in wheat seedlings.
Transcription factors controlling the wheat Cor /Lea gene expression
An ABA-independent wheat Cor gene, Wcor15, encodes a chloroplast-targeted COR protein analogous to the Arabidopsis protein COR15a (Lin and Thomashow 1992; Thomashow 1999; Takumi et al. 2003a). Expression of Wcs120 is also ABA-independent (Houde et al. 1992). The CRT/DRE-like sequence motifs are conserved in the promoter regions of the Wcor15 and Wcs120 genes, and their promoter sequences have been proven to be LT inducible in both monocotyledonous and dicotyledonous transgenic plants (Quellet et al. 1998; Takumi et al. 2003a). The Wdhn13 gene, which is responsive to both LT and drought, also contains the CRT/DRE motifs in its promoter region (our unpublished result). These results strongly suggest that the functional Cor /Lea gene system involving the CBF/ DREB1 trans -acting factors and the CRT/DRE cis element is conserved in wheat. Other conserved cis -acting elements such as ABRE and MYB- and MYC-binding motifs were also confirmed in the promoter regions of several wheat Cor /Lea genes such as Wdhn13, Wrab17, Wrab18 and Wrab19 (our unpublished results). The observation suggests that the wheat Cor /Lea genes are under control of the AREB (ABRE-binding factor), MYB and/ or MYC transcription factors through the ABA-dependent signal pathway, although it remains to be determined which elements are functional in cold-signal response.
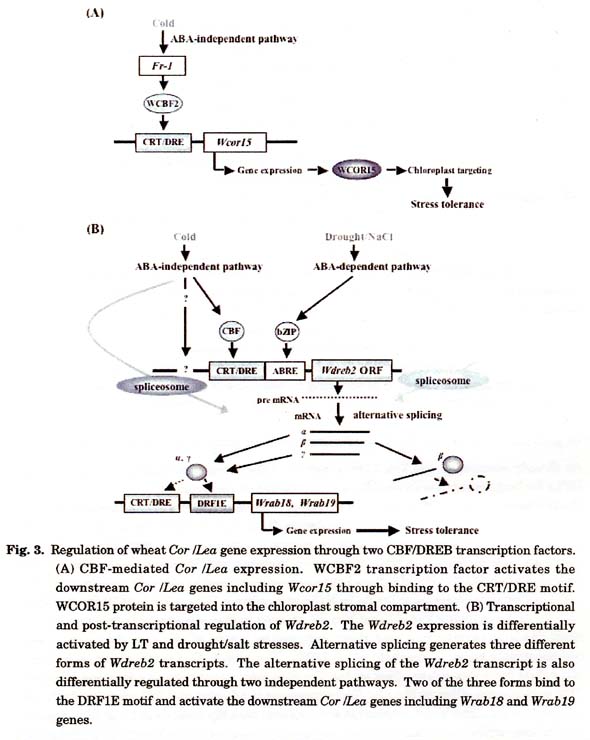
CBF/DREB1 transcription factors are known as key regulators of the cold signal transduction in various plant species (Thomashow 1999; Shinozaki and Yamaguchi Shinozaki 2000). Arabidopsis CBF/DREB1 transcripts start accumulating within 15 min after exposure to LT (Jaglo et al. 2001). Expression of the monocot CBF genes including barley HvCBF1 and HvCBF3 and rice OsDREB1A and OsDREB1B is also induced by LT within at least 2 h (Choi et al. 2002; Xue 2003; Dubouzet et al. 2003). HvCBF2, on the other hand, is constitutively expressed in barley leaves and its transcript level is rapidly enhanced by LT (Xue 2003). All Arabidopsis CBF /DREB1 genes except for CBF4 are activated by LT but not by salt, drought and exogenous ABA (Haake et al. 2002). Two Wcbf2 cDNA clones (accession numbers AB178166 and AB178167) that putatively encode wheat CBF/DREB1 homologs were isolated from cold-acclimated seedling leaves of M808 (Takumi et al. 2003b). The Wcbf2 gene and its homologs consist of a small multigene family. The Wcbf2 gene expression is responsive not only to LT but also to drought, and is involved in an ABA independent cold signal cascade in wheat (Kume et al. 2005). Throughout the cold acclimation period, M808 shows the higher levels of Wcbf2 transcript than CS. The overall expression profiles of the Wcbf2 gene showed a good correlation with development of freezing tolerance, strongly indicating that Wcbf2 plays an important role in the cold signal transduction and the development of freezing tolerance in wheat. The WCBF2 protein increased the GUS activities under control of the 5' upstream sequences of Wcor15 (Fig. 3A) and Wdhn13 in cultured cells of common wheat (Kume et al. 2005), indicating that WCBF2 directly functions as a transcriptional activator of these Cor /Lea genes. Further studies are required to prove the direct physical interaction of the CRT/DRE elements in these promoter regions with the WCBF2 protein. Nevertheless, these results suggest the important role of the CBF/DREB1 transcription factors in the predicted Cor /Lea gene signal transduction pathway in common wheat.
Arabidopsis DREB2 transcription factor also binds to the CRT/DRE motif and regulates the downstream Cor /Lea gene expression. The DREB2 transcript accumulates in response to drought but not to LT, and its expression level is controlled in an ABA-independent manner. Wheat TaDREB1 gene encodes an Arabidopsis DREB2 homolog, and the TaDREB1 protein binds to the CRT/DRE motif (Shen et al. 2003). The TaDREB1 gene is rapidly activated by LT, salt and ABA. Both TaDREB1 and the Wcs12O genes are induced rapidly and at relatively higher levels in winter wheat than in spring wheat. Another type of wheat DREB2 homolog, Wdreb2 (accession number AB193608), shows a high level of homology to barley HvDRF1 (our unpublished result), which binds preferably to a DRF1E motif, T(T/A)ACCGCCTT, rather than to the CRT/DRE motif (Xue and Loveridge 2004). The HvDRF1 gene is induced by drought and ABA, and HvDRF1 directly activates HVA1 expression. Similarly, the Wdreb2 transcript level is enhanced by LT, drought and ABA (our unpublished result). The Wdreb2 transcription is regulated by two independent pathways, i.e., an ABA-dependent pathway for drought and salt stresses and an ABA-independent pathway for cold stress. Like HvDRF1, three transcript forms of Wdreb2 were derived through alternative splicing (Fig. 3B). One of the splicing products encodes a non-functional polypeptide lacking ERF/AP2 and transcriptional activation domain (our unpublished results). The WDREB2 accumulation is thus differentially controlled at both the transcriptional and post-transcriptional levels under LT and drought/salt stress conditions in wheat. The DRF1E motif is present in the promoter regions of Wdhn13, Wrab18 and Wrab19 genes, and the expression of at least two Cor /Lea genes are directly activated by the WDREB2 transcription factor in wheat cell line (our unpublished results). The Wdreb2 gene therefore seemed to be a wheat ortholog of the barley HvDRF1 gene based on their high sequence homology and identical feature of alternative splicing. Wrab18 and Wrab19 showed high homology to barley HVA1 (Tsuda et al. 2000; Kobayashi et al. 2004a). Together with the presence of the DRF1E motif in the promoter region of Wrabl8 and Wrab19, it is indicated that they are wheat orthologs of HVA1. Thus, these stress signal pathways from Wdreb2 to Wrab18 /Wrab19 and from HvDRF1 to HVA1 were functionally conserved respectively in wheat and barley.
Barley Cor /Lea genes, HVA1 and HVA22, show ABA-induced gene expression through the ABA-response promoter complexes (ABRCs) (Shen et al. 2004). The ABRCs consist of an ACGT core motif (ABRE) and a coupling element. HvABI5 bZIP transcription factor recognizes the ABRCs and trans -activates its downstream genes (Casaretto and Ho 2003). This trans-activation is dependent on the presence of another transcription factor HvVP1. Some ABREs are found in the promoter regions of Wrab17, Wrab18 and Wrab19 genes (our unpublished results). A wheat ortholog of the barley HvABI5 was recently identified and designated as Wabi5 (accession number AB193553; our unpublished data). HvABI5 and WABI5 should function as transcription factors for some Triticeae Cor /Lea genes in the ABA-dependent pathway. Rice LIP19 gene encodes a bZLP-type transcription factor, and its expression is induced by LT (Aguan et al. 1993). Wheat LIP19 homolog, Wlip19 (accession number AB193552), shows also LT-inducible expression, suggesting that WLIP19 also recognizes the ABRE motif (our unpublished result). Although more detailed studies should be required to determine the direct interaction between the cis -elements and the transcription factors, the present information suggest the signal network responding to abiotic stresses in wheat, as shown in Fig. 4, which emphasizes differences from the Arabidopsis network. The DRF1E cis -element has been identified only in barley and wheat, and no cDNA clone has been obtained in Arabidopsis and rice yet. The HvDRF1/ WDREB2 are distinct from previously reported Arabidopsis DREB2 protein family at the level of amino acid sequence. It is tempting to determine if the interaction between DRE1E and HvDRF1/WDREB2-type transcription factor is specific to Triticeae.
Regulatory loci for the CBF-mediated Cor /Lea gene expression in wheat
As already mentioned, the Vrn-1 /Fr-1 regions of the group 5 chromosomes possess major QTLs for both winter hardiness (Fr) and vernalization requirement (Vrn). Substitution of the chromosome 5A of CS by that of a winter cv. 'Cheyenne' significantly increases freezing tolerance and COR/LEA protein accumulation (Limin et al. 1997; Danyluk et al. 1998). Similar relationships among vernalization response, Cor /Lea gene expression and freezing tolerance were shown using NILs of wheat cv. 'Norstar' and 'Manitou' for the Vrn-A1 locus (Danyluk et al. 2003). We also found the positive relationship between freezing tolerance and the Cor /Lea expression levels in NILs of a cultivar 'Triple Dirk' (TD) for the Vrn-1 loci: the NILs carrying Vrn-A1 and Vrn-B1 showed higher sensitivity to freezing damage than the non-carrier line and their expression levels of the Cor /Lea genes were comparatively lower (Kobayashi et al. 2005). The Vrn-1 /Fr-1 intervals in these Vrn-1 NILs thus have a negative effect on the Cor /Lea gene expression and freezing tolerance. This suppressive effect on the Cor /Lea expression is additive in a NIL carrying both Vrn A1 and Vrn-B1. The Wcbf2 transcript accumulated steadily until days 21 to 28 in the non-carrier line, while the transcript level was greatly reduced in the Vrn-1 NILs (Kobayashi et al. 2005). The Fr-1 or its closely linked loci in the Vrn-1 /Fr-1 intervals is thus probably responsible for regulating the CBF-mediated Cor /Lea gene expression in wheat (Fig. 3A and Fig. 4). The expression levels of Wdreb2 and Wlip19 were reduced to some extent in the Vrn-1 NILs, and the suppressive effect of Vrn-1 was additive (Kobayashi et al. 2004b). The level of Wcbf2 gene expression was more sensitive to the genotypes of the Vrn-1 /Fr-1 intervals than those of Wdreb2 and Wlip19. On the other hands, Wabi5 expression was not significantly affected by the genotype of the Vrn-1 /Fr-1 intervals (Kobayashi et al. 2004b), which is consistent with the ABA-independent nature of the Fr-1 function.

Neither positive nor negative relationships are found between the WAP1 gene expression and the Cbf2 /Cor /Lea gene expression (Kobayashi et al. 2005). The WAP1, which is a candidate of the Vrn-1 loci, is cold-responsive, and the level of WAP1 transcript is higher in a spring cultivar CS than in a winter cultivar M808 (Kobayashi et al. 2004b). A dramatic increase in the accumulation level of WAP1 mRNA was observed 12-24 hr after the LT treatment. This result clearly indicates that the WAP1 is not a regulator of Wcbf2 and other transcription factor genes, but rather suggests that loci controlling the cold/freezing adaptation in wheat are Fr-1. The Fr-1 loci play a critical role in regulation of the Wcbf2 expression level and at least partly control other transcription factor levels (Fig. 4). It can be envisioned that the Fr-1 loci represent the master loci functioning at the upstream of several cold-signal pathways that are mediated through several transcription factors regulating the expression of the Cor /Lea genes and thus freezing tolerance in wheat. The ABA-dependent pathway might be controlled by some other signal pathways rather than by the Fr-1 pathway, although the ABA-dependent pathway functions mainly in drought-stress response in wheat as well as in Arabidopsis (Shinozaki and Yamaguchi-Shinozaki 2000).
The lower levels of Wcbf2 and Cor /Lea transcripts and COR/LEA proteins and the lower freezing tolerance in the Vrn-1 NILs than the non-carrier line can be ascribed to the possession of the 'spring-type' Fr-1 loci by the NILs. On the other hand, the higher levels of Wcbf2 and Cor/Lea gene expression and freezing tolerance in the non-carrier line might result from the possession of the effective 'winter-type' Fr-1 alleles. It is generally expected that spring-type wheat possesses a 'spring-type' Fr-A1 allele linked with a dominant Vrn A1 allele in the Vrn-A1 /Fr-A1 interval. Oppositely, winter-type wheat should have a 'winter-type' Fr-A1 allele linked with a recessive vrn-A1 allele, reflecting the necessity of winter-type wheat to be equipped with the 'winter-type' Fr-A1 allele for attaining cold/ freezing tolerance. Our comparative expression studies between M808 and CS and among the TD NILs clearly indicate that the adaptive relationship between the Vrn-1 genotype and the Fr-1 genotype is conserved in wheat accessions. The Vrn-1 /Fr-1 intervals represent quite attractive chromosomal regions for understanding the genetic relationship between the major QTL loci and their adaptive evolution to environmental stresses. This adaptive relationship in Triticeae should be more carefully investigated in further studies.
Three barley CBF genes, HvCBF3, HvCBF4 and HvCBF8, are assigned to chromosome 5HL (Choi et al. 2002; Francia et al. 2004). However, the map location of the three HvCBF genes is considerably distant from the Vrn-H1 /Fr-H1 region and seems to be the same as that of the Fr-A2 locus assigned to chromosome 5AmL in T. monococcum (Vagujfalvi et al. 2003). The Fr-A2 seems to be orthologous to the Rcg1 (Regulator for cor14b gene) locus in common wheat (Vagujfalvi et al. 2000). The Rcg1 locus can regulate the expression level of wheat LT-inducible homolog of the barley cor14b (Vagujfalvi et al. 2000). The wheat sequence homologous to the HvCBF3 gene showed a tight linkage with the Fr-A2 QTL for frost tolerance on the T. monococcum map (Vagujfalvi et al. 2003). Chromosomal location of common wheat CBF/DREB1 homologs remains unknown, but the high homology of the Wcbf2 genes to the barley HvCBF genes suggests that Wcbf2 is probably located on the homoeologous group 5 chromosomes in the wheat genome. Relationship between Wcbf2 and Fr-A2 /Rcg1 should be clarified in a further study.
Role of ABA in cold acclimation in wheat
ABA regulates important aspects of plant growth and development, including environmental stress tolerance and seed maturation and dormancy (Leung and Giraudat 1998; Finkelstein et al. 2002). ABA is synthesized de novo mainly in response to drought and high salinity stresses (Shinozaki et al. 2003). Many genes as components of stress signaling pathways are induced by exogenous ABA in Arabidopsis and rice (Xiong et al. 2002a). Arabidopsis mutants, los (low expression osmotically responsive gene) 5/aba (ABA deficient) 3 and los6 /aba1, show severely reduced Cor /Lea gene expression under LT and reduced levels of freezing tolerance (Xiong et al. 2001, 2002b), suggesting that LT and ABA regulatory pathways are overlapped. Several Cor /Lea genes are in fact responsive to exogenous ABA, and their promoter sequences commonly contain ABRE (Lang and Palva 1992; Shinozaki and Yamaguchi-Shinozaki 2000). In wheat, the freezing tolerant winter cultivar M808 was more sensitive to exogenous ABA than the freezing susceptible spring cultivar CS at the seedling stage (our unpublished result). However, information on roles of ABA in regulation of the Cor /Lea and their transcription factor genes, cold acclimation and freezing tolerance is still limited. Since an ABA-hypersensitive mutant of a Japanese cultivar 'Chihoku-komugi' shows a higher level of freezing tolerance than the parental line (our unpublished results), the Arabidopsis information seems to be generally applicable to understanding possible roles of ABA in the wheat LT signal pathways.
An ABA-insensitive, non-dormant mutant line EH47-1 of common wheat was derived from EMS-induced mutagenesis of a highly ABA sensitive, dormant and red-grained line 'Kitakei-1354' (Kitakei) (Kawakami et al. 1997). In EH47-1, expression of ABA-responsive Cor /Lea genes is delayed after exogenous ABA treatment compared with that in Kitakei. Contrary to the expectation, however, the EH47-1 line showed a significantly higher level of freezing tolerance than Kitakei at least in the non-acclimated seedlings, although the mutation did not impair the cold acclimation ability per se of the mutant line (our unpublished results). The EH47-1 mutant allele has no influences on the expression levels of Cor /Lea genes under LT conditions (Fig. 4). Moreover, the higher freezing tolerance was apparently due to the elevated basal level of freezing tolerance in EH47-1. Although molecular mechanisms of the higher tolerance in EH47-1 still remain unknown, ABA sensitivity seem not to be associated with cold acclimation, while ABA is involved in determination of basal level of freezing tolerance in wheat.
Conclusion and perspective
Postulated LT signal pathways in wheat are illustrated in Fig. 4. LT signals mainly activate the CBF-regulon through Fr-1, and the Fr-1 loci seem to be ABA-independent in wheat. The CBF-mediated Cor /Lea gene expression plays central roles in cold acclimation to develop high levels of freezing tolerance. On the other hand, the ABA-dependent signal pathways might function to maintain the basal level of freezing tolerance. LT signals are transmitted to the transcription factors through ABA-dependent and -independent pathways. Several transcription factor genes coordinately regulate the accumulation levels of Cor /Lea genes. Whereas the Cor /Lea and transcription factor genes that take parts in the wheat LT signaling pathway are well corresponding to those in the model Arabidopsis, expression patterns of the transcription factor genes are partly different between them. Both ABA-dependent and -independent pathways in the cold/freezing tolerance seem to be distinct from the vernalization-related pathway involving the Vrn-1 loci. WAP1 expression is also LT-responsible and strongly regulated through the promoter and first intron regions (Murai et al. 2003; Yan et al. 2004; Fu et al. 2005). The molecular mechanisms of the LT-responsive WAP1 expression should be studied to further clarify the relationship between Vrn-1 and the Cor /Lea regulation. More importantly, molecular cloning of the Fr-1 loci is essential for understanding the LT signal network that directly controls freezing tolerance in wheat. The Vrn-1 /Fr-1 chromosomal intervals are significantly associated with several important aspects of wheat environmental adaptation. In the Vrn-Al /Fr-Al1intervals, winter-type wheat should possess at least one dominant 'winter-type' Fr-A1 allele that guarantees winter survival, but such allele is unnecessary for spring-type wheat (Storlie et al. 1998; Thomashow 1999). Such allelic linkage between the Vrn-1 and Fr-1 loci in the A genome is not necessarily observed in the D genome of Japanese common wheat landraces possessing Vrn-D1 or vrn-D1 allele (our unpublished results). Wheat and its related wild species carry large diversity of the cold/freezing tolerance levels. Comprehensive studies of allelic variation in Vrn-1 /Fr-1 intervals and the LT signal components should further facilitate our understanding of the molecular nature of the diversity in Triticum and Aegilops and help breeding wheat lines with superior abiotic stress tolerance.
Acknowledgments
We thank Drs. K. Mural, R. Ohno and F. Kobayashi for helpful discussion. We are also grateful to A. Torii and S. Hirosawa for technical supports in the cDNA-AFLP analysis. Contribution number 173 from the Laboratory of Plant Genetics, Faculty of Agriculture, Kobe University.
References
Aguan K, Sugawara K, Suzuki N and Kusano T (1993) Low-temperature-dependent expression of a rice gene encoding a protein with a leucine-zipper motif. Mol Gen Genet 240: 1-8.
Baker SS, Wilhelm KS and Thomashow MF (1994) The 5'-region of Arabidopsis thaliana cor15a has cis -acting elements that confer cold-, drought- and ABA-regulated gene expression. Plant Mol Biol 24: 701-713.
Baldi P, Grossi M, Pecchioni N, Vale G and Cattivelli L (1999) High expression level of a gene coding for a chloroplastic amino acid selective channel protein is correlated to cold acclimation in cereals. Plant Mol Biol 41: 233-243.
Brule-Babel AL and Fowler DB (1988) Genetic control of cold hardiness and vernalization requirement in winter wheat. Crop Sci 28: 879-884.
Casaretto JA and Ho THD (2003) The transcription factors HvABI5 and HvVP1 are required for the abscisic acid induction of gene expression in barley aleurone cells. Plant Cell 15: 271-284.
Cattivelli L, Baldi P, Crosatti C, Di Fonzo N, Faccioli P, Grossi M, Mastrangelo AM, Pecchionl N and Stanca AM (2002) Chromosome regions and stress-related sequences involved in resistance to abiotic stress in Triticeae. Plant Mol Biol 48: 649-665.
Chinnusamy V, Ohta M, Kanrar S, Lee B, Hong X, Agarwal M and Zhu JK (2003) ICE 1: a regulator of cold-induced transcriptome and freezing tolerance in Arabidopsis. Gene Develop 17: 1043-1054.
Choi DW, Zhu B and Close TJ (2002) Barley Cbf3 gene identification, expression pattern, and map location. Plant Physiol 129: 1781-1787.
Ciaffi M, Paolacci AR, Dominici L, Tanzarella OA and Porceddu E (2001) Molecular characterization of gene sequences coding for protein disulfide isomerase (PDI) in durum wheat (Triticum turgidum ssp. durum ).Gene 265: 147-156.
Danyluk J, Kane NA, Breton G, Limin AE, Fowler DB and Sarhan F (2003) TaVRT-1, a putative transcription factor associated with vegetative to reproductive transition in cereals. Plant Physiol 132:1849-1860.
Danyluk J, Perron A, Houde M, Limin A, Fowler B, Benhamou N and Sarhan F (1998) Accumulation of an acidic dehydrin in the vicinity of the plasma membrane during cold acclimation in wheat. Plant Cell 10: 623-638.
Dubouzet JG, Sakuma Y, Ito Y, Kasuga M, Dubouzet EG, Miura S, Seki M, Shinozaki K and Yamaguchi-Shinozaki K (2003) OsDREB genes in rice, Oryza sativa L., encode transcription activators that function in drought-, high-salt- and cold-responsive gene expression. Plant J 33: 751-763.
Finkelstein RR, Gampala SS and Rock CD (2002) Abscisic acid signaling in seeds and seedlings. Plant Cell 14 (Suppl): S15-S45.
Francia E, Rizza F, Cattivelli L, Stanca AM, Galiba G, Toth B, Hayes PM, Skinner JS and Pecchioni N (2004) Two loci on chromosome 5H determine low-temperature tolerance in a 'Nure' (winter) x 'Tremois' (spring) barley map. Theor Appl Genet 108:670-680.
Fu D, Szucs P, Yan L, Helguera M, Skinner JS, von Zitzewitz J, Hayes PM and Dubcovsky J (2005) Large deletions within the first intron in VRN-1 are associated with spring growth habit in barley and wheat. Mol Genet Genomics 273: 54-65.
Galiba G and Sutka J (1988) A genetic study of frost resistance in wheat callus culture. Plant Breed 101: 132-136.
Galiba G, Quarrie SA, Sutka J, Morgounov A and Snape JW (1995) RFLP mapping of the vernalization (Vrn1) and frost resistance (Fr1) genes on chromosome 5A of wheat. Theor Appl Genet 90: 1174-1179.
Grossi M, Giorni E, Rizza F, Stanca AM and Cattivelli L (1998) Wild and cultivated barleys show differences in the expression pattern of a cold-regulated gene family under different light and temperature conditions. Plant Mol Biol 38: 1061-1069.
Guy CL (1990) Cold acclimation and freezing stress tolerance: role of protein metabolism. Ann Rev Plant Physiol Plant Mol Biol 41: 187-223.
Guy CL, Niemi KJ and Brambl R (1985) Altered gene expression during cold acclimation of spinach. Proc Natl Acad Sci USA 82: 3673-3677.
Haake V, Cook D, Riechmann JL, Pineda 0, Thomashow MF and Zhang JZ (2002) Transcription factor CBF4 is a regulator of drought adaptation in Arabidopsis. Plant Physiol 130: 639-648.
Houde M, Danyluk J, Laliberte JF, Rassart E, Dhindsa RS and Sarhan F (1992) Cloning, characterization and expression of a CDNA encoding a 50-kilodalton.protein specifically induced by cold acclimation in wheat. Plant Physiol 99: 1381-1387.
Hsieh TH, Lee JT, Yang PT, Chiu LH, Charng YY, Wang YC and Chan MT (2002) Heterology expression of the Arabidopsis C-repeat/Dehydration response binding factor 1 gene confers elevated tolerance to chilling and oxidative stresses in transgenic tomato. Plant Physiol 129: 1086-1094.
Jaglo KR, Kleff S, Amundsen KL, Zhang X, Haake V, Zhang JZ, Deits T and Thomashow MF (2001) Components of the Arabidopsis C-repeat/dehydration responsive element binding factor cold- responsive pathway are conserved in Brassica napus and other plant species. Plant Physiol 127: 910-917.
Kawakami N, Miyake Y and Noda K (1997) ABA insensitivity and low ABA levels during seed development of non-dormant wheat mutant. J Exp Bot 48: 1415-1421.
Kobayashi F, Takumi S, Nakata M, Ohno R, Nakamura T and Nakamura C (2004a) Comparative study of the expression profiles of the Cor /Lea gene family in two wheat cultivars with contrasting levels of freezing tolerance. Physiol Plant 120: 585-594.
Kobayashi F, Takumi S and Nakamura C (2004b) Regulation of cold-responsive Cor /Lea genes and their transcription factors by the major freezing tolerance locus Fr-1 in wheat. Rec Res Devel Plant Sci 2: 249-266.
Kobayashi F, Takumi S, Kume S, Ishibashi M, Ohno R, Murai K and Nakamura C (2005) Regulation by Vrn-1 /Fr-1 chromosomal intervals of CBF-mediated Cor /Lea gene expression and freezing tolerance in common wheat. J Exp Bot 56: 887-895.
Kume S, Kobayashi F, Ishibashi M, Ohno R, Nakamura C and Takumi S (2005) Differential and coordinated expression of Cbf and Cor / Lea genes during long-term cold acclimation in two wheat cultivars showing distinct levels of freezing tolerance. Genes Genet Syst 80: 185-197.
Lang V and Palva ET (1992) The expression of a rab -related gene, rabi8, is induced by abscisic acid during the cold acclimation process of Arabidopsis thaliana (L.) Heynh. Plant Mo! Biol 20: 951- 962.
Law CN and Jenkins G (1970) A genetic study of cold resistance in wheat. Genet Res Camb 15: 197- 208.
Leung J and Giraudat J (1998) Abscisic acid signal transduction. Ann Rev Plant Physiol Plant Mol Biol 49: 199-222.
Levitt J (1980) Responses of plants to environmental stresses. Vol. 1 (2nd edn.), Academic Press, New York, pp166-222.
Limin AE, Danyluk J, Chauvin LP, Fowler DB and Sarhan F (1997) Chromosome mapping in low- temperature induced Wcs12O family genes and regulation of cold-tolerance expression in wheat. Mol Gen Genet 253: 720-727.
Lin C and Thomashow MF (1992) DNA sequence analysis of a complementary DNA for cold-regulated Arabidopsis COR15 and characterization of the COR15 polypeptide. Plant Physiol 115: 171- 180.
Liu Q, Sakuma Y, Abe H, Kasuga M, Miura S, Yamaguchi-Shinozaki K and Shinozaki K (1998) Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain, separate two cellular signal transduction pathways in drought- and low temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell 12: 165-178.
Murai K, Miyamae M, Kato H, Takumi S and Ogihara Y (2003) WAP1, a wheat APETALA1 homolog, plays a central role in the phase transition from vegetative to reproductive growth. Plant Cell Physiol 44: 1255-1265.
Narusaka Y, Nakashima K, Shinwari ZK, Sakuma Y, Furihata T, Abe H, Narusaka M, Shinozaki K and Yamaguchi-Shinozaki K (2003) Interaction between two cis -acting elements, ABRE and DRE, in ABA-dependent expression of Arabidopsis rd29A gene in response to dehydration and high-salinity stresses. Plant J 34: 137-148.
NDong C, Danyluk J, Wilson KE, Pocock T, Huner NPA and Sarhan F (2002) Cold-regulated cereal chloroplast late embryogenesis abundan-like proteins. Molecular characterization and functional analysis. Plant Physiol 129: 1368-1381.
Novillo F, Alonso JM, Ecker JR and Salinas J (2004) CBF2/DREB1C is a negative regulator of CBF1 / DREB1B and CBF3 IDREB1A expression and plays a central role in stress tolerance in Arabidopsis. Proc Natl Acad Sci USA 101: 3985-3990.
Ohno R, Takumi S and Nakamura C (2001) Expression of a cold-responsive Lt-Cor gene and development of freezing tolerance during cold acclimation in wheat (Triticum aestivum L.). J Exp Bot 52: 2367-2374.
Ohno R, Takumi S and Nakamura C (2003) Kinetics of transcript and protein accumulation of a low- molecular-weight wheat LEA D-11 dehydrin in response to low temperature. J Plant Physiol 160: 193-200.
Pan A, Hayes PM, Chen F, Chen THH, Blake T, Wright S, Karsai I and Bedo Z (1994) Genetic analysis of the components of winter hardiness in barley (Hordeum vulgare L.). Theor Appl Genet 89: 900-910.
Pearce RS, Houlston CE, Atherton KM, Rixon JE, Harrison P, Hughes MA and Dunn MA (1998) Localization of expression of three cold-induced genes, blt101, blt4.9, and blt4, in different tissues of the crown and developing leaves of cold-acclimated cultivated barley. Plant Physiol 117: 787- 795.
Quellet F, Vazquez-Tello A and Sarhan F (1998) The wheat wcs12O promoter is cold-inducible in both monocotyledonous and dicotyledonous species. FEBS Let 423: 324-328.
Rabbani MA, Maruyama K, Abe H, Khan MA, Katsura K, Ito Y, Yoshiwara K, Seki M, Shinozaki K and Yamaguchi-Shinozaki K (2003) Monitoring expression profiles of rice genes under cold, drought, and high-salinity stresses and abscisic acid application using cDNA microarray and RNA gel-blot analyses. Plant Physiol 133: 1755-1767.
Roberts DWA (1986) Chromosomes in 'Cadet' and 'Rescue' wheats carrying loci for cold hardiness and vernalization response. Can J Genet Cytol 28: 991-997.
Seki M, Narusaka M, Ishida J, Nanjo T, Fujita M, Oono Y, Kamiya A, Nakajima M, Enju A, Sakurai T, Satou M, Akiyama K, Taji T, Yamaguchi-Shinozaki K, Carninci P, Kawai J, Hayashizaki Y and Shinozaki K (2002) Monitoring the expression profiles of 7000 Arabidopsis genes under drought, cold and high-salinity stresses using a full-length eDNA microarray. Plant J 31: 279- 292.
Shen YG, Zhang WK, He SJ, Zhang JS, Liu Q and Chen SY (2003) An EREBP/AP2-type protein in Triticum aestivum was a DRE-binding transcription factor induced by cold, dehydration and ABA stress. Theor Appl Genet 106: 923-930.
Shen QJ, Casaretto JA, Zhang P and Ho THD (2004) Functional definition of ABA-response complexes: the promoter units necessary and sufficient for ABA induction of gene expression in barley (Hordeum vulgare L.). Plant Mol Biol 54: 111-124.
Shimamura C, Ohno R, Nakamura C and Takumi (2005) Improvement of freezing tolerance in transgenic tobacco plants with a chloroplast-targeting and cold-responsive protein WCOR15 of common wheat. J Plant Physiol (accepted).
Shinozaki K and Yamaguchi-Shinozaki K (2000) Molecular responses to dehydration and low temperature: differences and cross-talk between two stress signaling pathways. Curr Opin Plant Biol 3: 217-223.
Shinozaki K, Yamaguchi-Shinozaki K and Seki M (2003) Regulatory network of gene expression in the drought and cold responses. Curr Opin Plant Biol 6: 410-417.
Snape JW, Sarma R, Quarrie SA, Fish L, Galiba G and Sutka J (2001) Mapping genes for flowering time and frost tolerance in cereals using precise genetic stocks. Euphytica 120: 309-315.
Snape JW, Semikhodskii A, Fish L, Sarma RN, Quarrie SA, Galiba G and Sutka J (1997) Mapping frost tolerance loci in wheat and comparative mapping with other cereals. Acts Agron Hung 45: 265-270.
Stockinger EJ, Gilmour SJ and Thomashow MF (1997)Arabidopsis thaliana CBF1 encodes an AP2 doomain-containing transcription activator that binds to the C-repeat/DRE, a cis -acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proc Natl Acad Sci USA 94: 1035-1040.
Storlie EW, Allan RE and Walker-Simmons MK (1998) Effect of the Vrn1-Fr1 interval on cold hardiness levels in near-isogenic wheat lines. Crop Sci 38: 483-488.
Sutka J (1981) Genetic studies of frost resistance in wheat. Theor Appl Genet 59: 145-152.
Sutka J (2001) Genes for frost resistance in wheat. Euphytica 119: 167-172.
Sutka J and Snape JW (1989) Location of a gene for frost resistance on shromosome 5A of wheat. Euphytica 42: 41-44.
Sutka J, Galiba G, Vagujfalvi A, Gill BS and Snape JW (1999) Physical mapping of the Vrn-A1 and Fr1 genes on chromosome 5A of wheat using deletion lines. Theor Appl Genet 99: 199-202.
Takumi S, Koike A, Nakata M, Kume S, Ohno R and Nakamura C (2003a) Cold-specific and light stimulated expression of a wheat (Triticum aestivum L.) Cor gene Wcor15 encoding a chloroplast-targeted protein. J Exp Bot 54: 2265-2274.
Takumi S, Ohno R, Kobayashi F, Nakata M, Ishibashi M, Kume S, Egawa C, Shimamura C, Nakamura T and Nakamura C (2003b) Cultivar differences in cold acclimation/freezing tolerance and Cor gene expression in common wheat. Proc X Int Wheat Genet Symp Paestum 3: 1269-1271.
Thomashow MF (1999) Plant cold acclimation: freezing tolerance genes and regulatory mechanisms. Ann Rev Plant Physiol Plant Mol Biol 50: 571-599.
Toth B, Galiba G, Feher E, Sutka J and Snape JW (2003) Mapping genes affecting flowering time and frost resistance on chromosome 5B of wheat. Theor Appl Genet 107: 509-514.
Trevaskis B, Bagnall DJ, Ellis MH, Peacocck WJ and Dennis ES (2003) MADS box genes control vernalization-induced flowering in cereals. Proc Natl Acad Sci USA 100: 13099-13104.
Tsuda K, Tsvetanov S, Takumi S, Mon N, Atanassov A and Nakamura C (2000) New members of a cold-responsive group-3 Lea /Rab -related Cor gene family from common wheat (Triticum aestivum L.). Genes Genet Syst 75: 179-188.
Tsvetanov S, Ohno R, Tsuda K, Takumi S, Mon N, Atanassov A and Nakamura C (2000) A cold responsive wheat (Triticum aestivum L.) gene wcor14 identified in a winter-hardy cultivar 'Mironovska 808'. Genes Genet Syst 75: 49-57.
Vagujfalvi A, Crosatti C, Galiba G, Dubcovsky J and Cattivelli L (2000) Two loci on wheat chromosome 5A regulate the differential cold-dependent expression of the cor14b gene in frost-tolerant and frost-sensitive genotypes. Mol Gen Genet 263: 194-200.
Vagujfalvi A, Galiba G, Cattivelli L and Dubcovsky J (2003) The cold-regulated transcriptional activator Cbf3 is linked to the frost-tolerance locus Fr-A2 on wheat chromosome 5A. Mol Genet Genomics 269: 60-67.
Veisz O and Sutka J (1989) The relationships of hardening period and expression of frost resistance in chromosome substitution lines of wheat. Euphytica 43:41-45.
Veisz O and Sutka J (1990) Frost resistance studies with wheat in natural and artificial conditions. In: Panayotov I and Pavlova S (eds.) Proceedings of International Symposium on Cereal Adaptation to Low Temperature Stress, Albena, pp. 12-17.
Xiong L, Ishitani M, Lee H and Zhu JK (2001) The Arabidopsis LOS5 /ABA3 locus encodes a molybdenum cofactor sulfurase and modulates cold stress- and osmotic stress-responsive gene expression. Plant Cell 13: 2063-2083.
Xiong L, Schumaker KS and Zhu JK (2002a) Cell signaling during cold, drought, and salt stress. Plant Cell 14 (Suppl): S165-S183.
Xiong L, Lee H, Ishitani M and Thu JK (2002b) Regulation of osmotic stress-responsive gene expression by the LOS6 /ABA1 locus in Arabidopsis. J Biol Chem 277: 8588-8596.
Xue GP (2003) The DNA-binding activity of an AP2 transcriptional activator HvCBF2 involved in regulation of low-temperature responsive genes in barley is modulated by temperature. Plant J 33: 373-383.
Xue GP and Loveridge CW (2004) HvDRF1 is involved in abscisic acid-mediated gene regulation in barley and produces two forms of AP2 transcriptional activators, interacting preferably with a CT-rich element. Plant J 37: 326-339.
Yamaguchi-Shinozaki K and Shinozaki K (1994) A novel cis -acting element in an Arabidopsis gene is involved in responsiveness to drought, low-temperature, or high salt stress. Plant Cell 6: 251 264.
Yan L, Loukoianov A, Tranquilli G, Helguera M, Fahima T and Dubcovsky J (2003) Positional cloning of the wheat vernalization gene VRN1. Proc Natl Acad Sci USA 100: 6263-6268.
Yan L, Helguera M, Kate K, Fukuyama S, Sherman J and Dubcovsky J (2004) Allelic variation at the VRN-1 promoter region in polyploidy wheat. Theor AppI Genet 109: 1677-1686.
Zarka DG, Vogel JT, Cook D and Thomashow MF (2003) Cold induction of Arabidopsis CBF genes involves multiple ICE (inducer of CBF expression) promoter elements and a cold-regulatory circuit that is desensitized by low temperature. Plant Physiol 133: 910-918.