Memorial Issue, Wheat Information Service No.100
Gametocidal genes in wheat as the inducer of chromosome breakage
Hisashi Tsujimoto
Laboratory of Plant Genetics and Breeding Science, Faculty of Agriculture,
Tottori University, Tottori 680-8553, Japan Key words: gametocidal gene, chromosome aberration, alien
chromosome addition line,restriction-modification, selfish genetic element,
Aegilops species, Triticum aestivum
Introduction
In the process of recurrent backcrossing to produce alloplasmic lines or alien
chromosome addition lines, some alien chromosomes transmit preferentially to
the offspring. These chromosomes have a gametocidal (Gc) gene(s) that kill gametes
lacking it in the monosomic addition states. The alien chromosomes carrying
the Gc gene are termed 'gametocidal' (Endo 1979) or 'cuckoo' chromosomes (Miller
1983). It is known that the Gc genes kill gametes by breaking the chromosomes
(Finch et al. 1984; Tsujimoto et al. 1990; Nasuda et al.
1998). However, the molecular mechanism of this phenomenon has not yet been
clarified. Many chromosome deletions and translocations have been produced in
wheat chromosomes (Endo and Gill 1996;.Tsujimoto et al. 2001) and the
alien chromosomes added to the wheat genetic background using the action of
Gc genes (Friebe et al. 2000; Shi and Endo 2000). These wheat strains
have made it possible to physically map genes, ESTs, and markers to specific
regions on wheat and barley chromosomes (Qi et al. 2004; Nasuda et
al. 2005). After previous review by Endo (1990) several remarkable findings
about Gc genes were reported. In addition, the techniques of molecular genetics
have advanced considerably in these 15 years. Toward revelation of the molecular
mechanisms of chromosome breakage by the Gc genes, the genetical features of
the Gc genes are reviewed here.
Gametocidal genes are selfish genetic factors
The basic nature of the Gc genes appears in gametogenesis in the alien monosomic
addition plants. Both male and female gametes without the Gc gene become abortive,
and as a result, only gametes with the Gc gene (and thus, with the alien chromosome)
are transmitted to the next generation. Gc action in the female germline manifests
as sporadic seed sets on spikes, whereas in the male germline, it appears as
a mixture of normal and abortive pollen grains. Homozygotes for the Gc gene
(that is, disomic alien chromosome addition lines) do not show such gametic
abortion, like normal plant without the Gc gene, because all of the gametes
carry the alien chromosome with Gc gene.
Several species of the genus Aegilops carry a
Gc gene that is located on chromosome 3C of Aegilops triuncialis
(genome formula CCUU) and Ae. caudata
(CC, Endo and Tsunewaki 1975); chromosome 2C of Ae. cylindrica
(CCDD, Endo 1979); chromosome 4Sl
or 2Sl of Ae. longissima
(SlSl) and
Ae. sharonensis (SlSl,
Maan 1975; Endo 1985); chromosome 2S or 6S of Ae. speltoides
(SS, Tsujimoto and Tsunewaki 1984, 1988; Kota and Dvorak 1988); or
chromosome 4Mg of Ae. geniculata
(syn. Ae. ovata UUMM, Kynast et
at. 2000). Ae. sharonensis strain KU5-1
carries two Gc genes on both chromosomes 2Sl
and 4Sl (Tsujimoto 1994). All of the diploid
progenitor species possessing Gc genes tend to be out, breeding in nature. Once
a Gc gene is created by mutation or has immigrated from other populations, it
will rapidly increase in frequency by preferential transmission. The Gc gene
will soon be fixed in the population and will lurk in the genome, because the
Gc genes lose selective advantage. In this sense, the Gc gene is highly selfish
and parasitic to the species. It is not known, however, if Gc genes are functional
in their species of origin. Kihara (1959) produced alloplasmic lines with Ae.
caudata cytoplasm without the Gc chromosome. Feldman (1979) produced
a series of seven addition lines for all of Ae. longissima
chromosomes. Friebe et at. (1992) reported seven
addition lines of wheat with a pair of Ae. caudata chromosome.
These facts may indicate that the strains of the Aegilops
species did not carry the Gc gene. However, it is known that some
cultivars of common wheat possess a gene that suppresses the function of the
Gc gene. If the cultivars with the suppressor were used as the nucleus donors
of the alloplasimc lines or the recipients of the addition chromosomes, the
Gc gene would not have been noticed. Moreover, if the alien species has the
suppressor, Gc will be removed in the early backcross generations.
E-mail address: tsujim@muses.tottori-u.ac.jp
Suppressor of gametocidal gene
Endo (1978) reported that monosomic addition of chromosome 3C of Ae. triuncialls showed both male and female semi-sterility in the genetic backgrounds of the common wheat cultivars 'Jones Fife' (abbrev. JF) and 'Chinese Spring' (abbrev. CS). However, semi-sterility did not appear in the cultivar 'Norin 26' (abbrev. N26). Chromosome 3C preferentially transmitted to the next generation from both sides in JF but only from the female side in CS. Although Endo (1978) did not mention transmission of Gc chromosome in N26, recovery of fertility in the genetical background suggested that chromosome 3C transmitted normally as usual alien monosome without Gc.
Tsujimoto and Tsunewaki (1985a) analyzed the genetic factor in N26 that suppresses the Gc function of chromosome 3C. They crossed the disomic addition line of CS carrying chromosome 3C (abbrev.: CS+3C3C) with the F1 progeny of CS and N26. In the monosomic addition lines, fertile and semi-sterile plants segregated 1:1. The data indicated that a dominant suppressor gene (Igc1) controls the suppression of Gc gene action on chromosome 3C. By monosomic analysis, Igc1 was localized to chromosome 3B of N26. Moreover, pollen carrying Igc1 had a slight advantage during fertilization over pollen carrying igc1. The facts that both the Gc gene and the suppressor were located on chromosomes of the same homoeologous group and that Igc1 is located in the B genome that originated from an outcrossing species together suggest that Igc1 also has Gc properties.
In the JF genetic background, both male and female gametes without
chromosome 3C were abortive, whereas in the CS background, pollen without the
Gc chromosome functioned and transmitted to the progeny. This result suggested
the existence of an incomplete suppressor in the CS background, though a detailed
analysis has not yet been carried out. In addition, Igc1
can not suppress the action of Gc genes in Ae. sharonensis,
Ae. longissima,or Ae. speltoides. No
suppressors for the Gc actions of Ae. longissima, Ae. sharonensis,
or Ae. speltoides chromosomes were discovered
among the hundreds of common wheat cultivars tested so far (Tsujimoto, unpub.).
Chromosome amplification by Igc1
To learn the position of Igc1 on the chromosome 3B, telosomic analysis was conducted. First, N26 was crossed with ditelocentric 3BS (abbrev. dt3BS) or 3BL (dt3BL) of CS, and then the F1 was crossed with CS+3C3C. Both the chromosome constitution and the self. fertility of plants were observed (Table 1). Fertility segregated clearly, and plants with more than 65% fertility were regarded as 'fertile' and less than 35% fertility as 'semi-sterile'. No intermediate plants appeared. In a cross with dt3BL, most of the plants carried the expected chromosome constitution, 2n=42+3C or 41+3BL+3C. However, in a cross with dt3BS, 52 of 94 plants (55%) showed an unexpected chromosome constitution. Many of the unexpected plants carried one or two extra chromosomes. C-banding analysis and meiotic chromosome configuration indicated that most of the extra chromosomes were chromosome 3B (Fig. 1). This strange phenomenon of chromosome amplification appeared in five independent crosses in which four different F1 plants (CS x dt3BS) were used. This phenomenon also reappeared in plants crossed the next year. Because the hybrids using CS or CS+2C2C of Ae. cylindrica instead of CS+3C3C do not induce this phenomenon, combination between Igc1 and chromosome 3C is the cause of chromosome amplification.
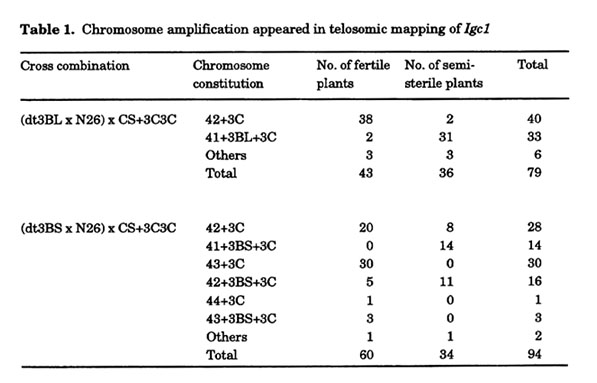
In the cross with dt3BL, two of 40 2n=42+3C plants were semi-sterile, and two of the33 2n=41+3BL+3C plants were fertile. These plants maybe recombinants. However, more plants in the cross with dt3BS seemed to have a recombined chromosome. In this cross combination, eight of 28 2n=42+3C plants and five of 16 2n=42+3BS+3C plants carried a recombined chromosome, despite the fact that no recombined chromosomes appeared in 2n=41+3BS+3C or 43+3C plants. The correlation between chromosome constitution and fertility hints at a possible mechanism of chromosome amplification involving the Gc gene and Igc1; however, further investigation is necessary to fully understand the phenomenon.
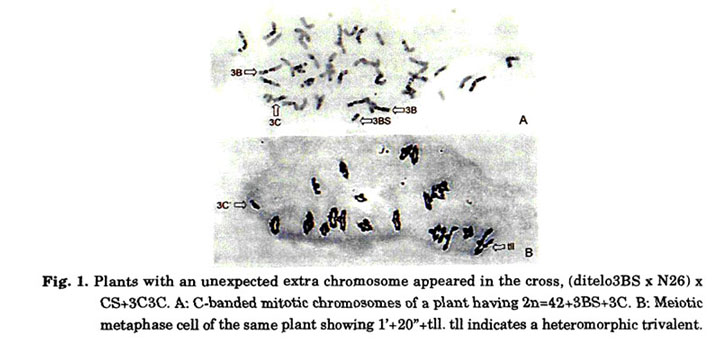
Distribution of Igc1 in wheat cultivars
Tsujimoto and Tsunewaki (1985a) crossed 36 cultivars of common wheat with CS+3C3C and classified them into Igc1 carriers and non-carriers based on the seed fertility of the F1 progeny. Igc1 exists in Japanese cultivars but not in American or African cultivars. Tsujimoto et al. (1998) examined the distribution of Igc1 in additional cultivars originating from Asia and other regions. Igc1 is detectable in cultivars from Southwest Japan, East China, and Southwest China (Sichuan). However, Igc1 is not found in cultivars from Northeast China, Inner Mongolia, Xinjiang, Tibet, Bhutan, Nepal, Afghanistan or more western regions (Fig. 2). In the boundary regions (that is, Northwest China, Korea, and Northeast Japan) both types were detected. Yen et al. (1988) reported that CS is a landrace in Sichuan most similar to 'Chendu-guang-tou' in the Sichuan Basin. However, CS is an Igc1 non-carrier in spite of the fact that most Sichuan landraces are carriers. This suggests that CS, and possibly 'Chendu-guang-tou' as well, originated from mountainous regions of western Sichuan where the Tibetan type landraces are cultivated. The Japanese cultivar 'Norin 10', which was used as the source of semi-dwarf genes Rht1 and Rht2 in modern wheat breeding, carried Igc1. Since historic American cultivar 'Gaines' bred by crosses including 'Norin 10' does not carry Igc1, the distribution of Igc1 was still strictly remained only in the eastern Asian wheat cultivars. The frequency of Igc1 in these regions was extremely high, or in the other words, cultivars in these regions can be characterized by the presence of Igc1. The high frequency of Igc1 in these regions may be attributable to either selective advantage of Igc1 or a bottleneck effect that occurred during the dispersion of common wheat from a western region to eastern China. Although pollen with Igc1 participated more frequently in fertilization, it is not enough to explain the almost exclusive existence of Igc1 in east Asia.
Chromosome breakage by gametocidal genes of Ae. triuncialis and Ae. cylindrica
The F1 hybrids between N26 and CS+3C3C had the expected mitotic chromosome constitution, 2n=42+3C. These hybrids underwent meiosis normally, showing 21 bivalents of wheat and one univalent of chromosome 3C in the first metaphase. Back-crossed or self-pollinated progeny of the F1, however, contained many plants with one or more aberrant chromosomes. The aberrations included telocentric, acrocentric, deletion, dicentric, ring, and translocated chromosomes (Tsujimoto and Tsunewaki 1985a, Fig. 3). Since such abnormal chromosomes did not appear in the offspring of F2 between N26 and CS, chromosome 3C must be the cause of breakage induction. With the exception of unstable dicentric and ring chromosomes, the broken chromosomes of root tip cells were not chimeric, indicating that the breakage occurred in gametes or zygotic cells and then stabilized before the first cell division.
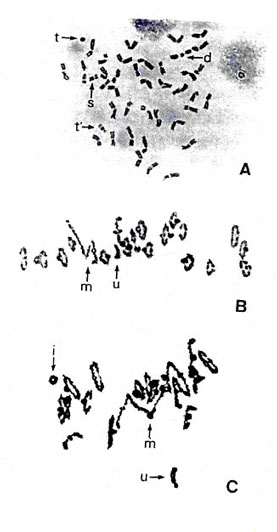

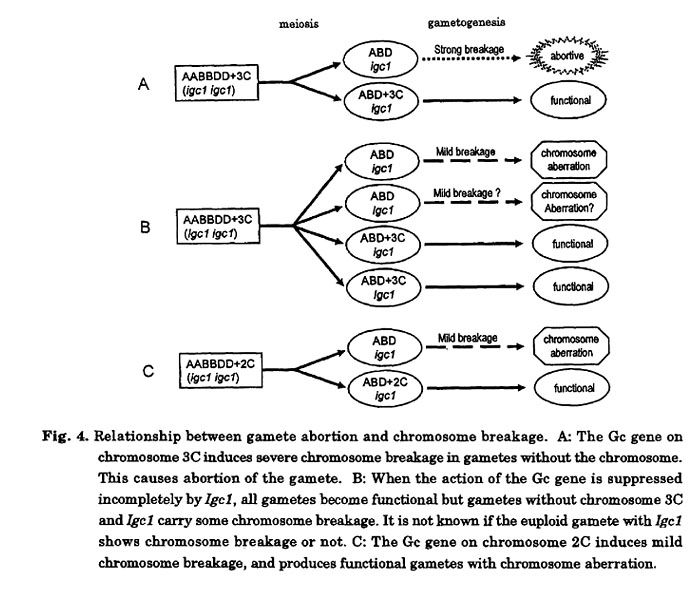
A similar phenomenon was observed in the addition line of CS with chromosome 2C of Ae. cylindrica (Endo 1988a). The chromosome exhibited the characteristics of a Gc chromosome in a JF genetic background (Endo 1979) but not in a CS background. The disappearance of Gc action in CS is similar to the case of chromosome 3C from Ae. triuncialis in a N26 background, although the suppressor of CS for chromosome 2C has not been identified. Chromosome aberrations caused by chromosome 2C appeared most often in offspring without the alien chromosome. Endo (1988a) suggested that when the gametocidal action is intense, gametophytes without the alien chromosome may suffer severe chromosome breakage and become sterile (Fig. 4A), ensuring exclusive transmission of the alien chromosome. When the gametocidal action is mild, gametophytes without the alien chromosome are fertilized, suffering slight chromosome damage, and develop into plants with chromosome aberrations (Fig. 4B, C).
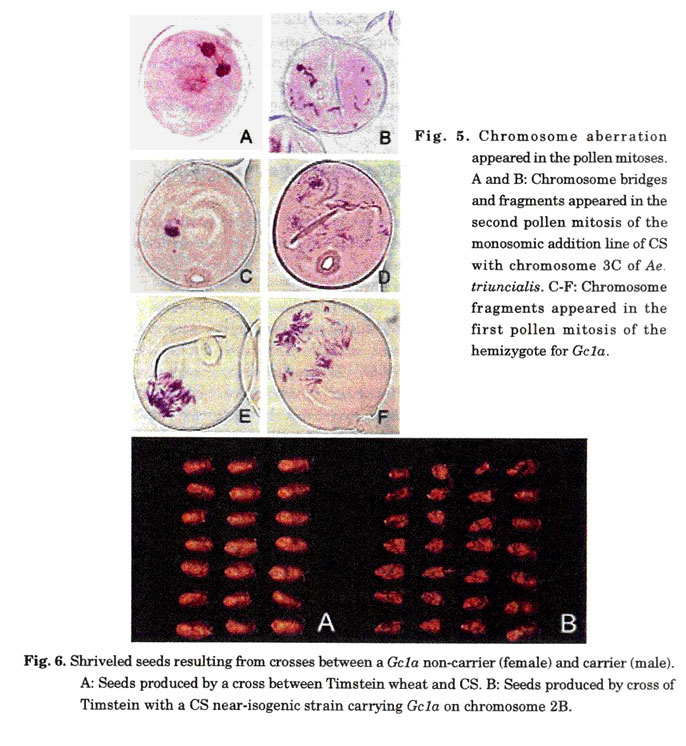
Tsujimoto et al. (1990) observed chromosome fragments, bridges, and micronuclei in the first and the second pollen mitoses of CS+3C (Fig. 5A, B). Nasuda et al. (1998) observed similar chromosome aberrations in CS+2C. The data provide direct evidence for chromosome breakage in microsporogenesis. Observation of cell divisions during gametogenesis in female plants is difficult However, Tsujimoto et al. (2001) predicted that breakage during female gametogenesis occurs from the following experiment. To isolate deletions in chromosome 1B, they crossed CS+2C (female) to nullisomic 1B-tetrasomic 1D of CS (male) at a large scale. Next, they cut the F1 seeds into halves. The halves without the embryo were used to examine the storage proteins produced by genes located on the short and long arms of the chromosome 1B. In this study, They found that the genotype of the endosperm did not always coincide with the genotype of the embryo in the same seed, and concluded that the chromosomes are broken during cell divisions in female gametogenesis.
The breakpoints of the chromosome aberrations do not appear to be distributed randomly in wheat chromosomes and may be restricted to specific chromosome structures or DNA sequences (Endo and Gill 1996). Tsujimoto et al. (2001) recognized breakage 'hot spots' in their effort to produce a large-scale collection of 1B deletion chromosomes. However, the distribution pattern of the breakage hot spots in these two studies did not coincide with each other, despite the fact that both studies used the same Gc gene (Tsujimoto et al. 2001; Friebe et al. 2001). The broken end gradually acquired telomere repetitive sequences, indicating that incomplete (or perhaps undetected) telomere sequences were enough to heal the broken ends (Tsujimoto 1993). Using the telomere sequence as a primer for PCR, the DNA sequences at the broken ends were amplified and then analyzed. However, no specific sequences were observed (Tsujimoto et al. 1997, 1999).
The abnormal chromosomes induced by the Gc gene of Ae.triuncialis or Ae. cylindricacan be transmitted to the next generation. Because the breakage occurs only in the gametes without Gc chromosome in monosomic addition lines, the strains with a chromosome deletion in the next generation were stable and did not induce additional chromosome aberrations. Thus, these deletion lines were useful for mapping and were maintained as the standard for mapping genes to specific chromosome regions (Endo and Gill 1996; Tsujimoto et al. 2001).
Chromosome breakage and mutation by gametocidal genes of Ae.longissima, Ae. sharonensis and Ae. speltoides
The Gc genes of Ae. longissima, Ae. sharonensis, and Ae. speltoides also induce chromosome breakage (Tsujimoto and Tsunewaki 1985b; Tsujimoto and Noda 1989; Endo 1988b). However, as mentioned above, the mode of their action of chromosome breakage differs from that of Gc genes in Ae. triuncialis and Ae.cylindrica. The Gc genes in Ae. longissima, Ae. sharonensis or Ae. speltoides can cause breakage in the gametes like those of Ae. triuncialis and Ae. cylindrica. Finch et al. (1984) observed chromosome breakage and gaps in the pollen mitoses in a monosomic addition line with chromosome 4Sl from Ae. sharonensis. Similarly, Nasuda et al. (1998) observed chromosome breakage in a line with the Gc gene of Ae. speltoides (Fig. 5C-F, original figure). These chromosomal abnormalities seemed to occur specifically in gametes lacking the Gc gene. Friebe et al. (2003) demonstrated directly that chromosome breakage in pollen mitosis occurred only in gametes lacking the Gc gene by fluorescent in situ hybridization with a probe of a repetitive DNA sequence that marks the Gc gene.
The Gc genes of these species also induce chromosome breakage in zygotic cells. Endo (1988b) reported chromosome breakage in the F1 progeny of a cross between CS or monosomic 4B and disomic addition lines with chromosome 4Sl of Ae. sharonensis or Ae. longissima. Since mutations occurred more frequently when the monosomic plant was the female, chromosome 4B in the egg cell may partially suppress chromosome breakage (Endo 1988b). King and Laurie (1993) observed chromosome aberration in early zygotic and endosperm cells of the F1 progeny of monosomic 4B (female) crossed with the substitution line of the chromosome 4Sl for 4B (male).
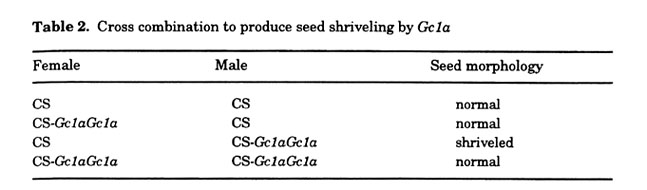
Tsujimoto and Tsunewaki (1984, 1988) identified two Gc genes, Gc1a and Gc1b, from Ae. speltoides. These genes have been naturally transferred independently to chromosome 2B of common wheat (Tsujimoto and Tsunewaki 1984, 1988). Homozygotes for the Gc genes showed normal fertility because all of the gametes carried the Gc gene. The near isogenic line (NIL) of CS with Gc1a was morphologically the same as CS without Gc1a,except for a slight reduction of fertility from 99.3 to 96.5% (Tsujimoto and Tsunewaki 1983). However, when NIL of Gc1a (male) was crossed with a normal CS strain or another cultivars without Gc1a, shriveled seeds that germinate at low frequency appeared often, though the effect was dependent on exposure to low temperature after fertilization (Tsujimoto and Tsunewaki 1985b, 1985c, 1988). The reciprocal cross, as well as self-pollination, did not produce shriveled seeds (Fig. 6, Table 2). Moreover, spikes from plants originating from shriveled seeds often showed the same abnormal morphology. Neither Gc1b nor the other Gc genes of Ae. longissima and Ae. sharonensis showed such seed shriveling. However, they did cause chromosomal mutations and phenotypic changes in the F1 generation (Tsujimoto and Tsunewaki 1985b, unpublished data).
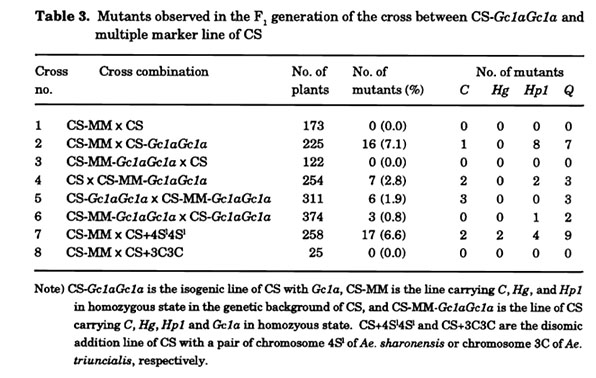
To better understand the genetical property of the mutation(s) that occurred in the F1 progeny of Gc1a carrier, Tsujinioto and Noda (1990) asked what results from cross of the multiple marker line of cultivar 'S-615' (abbrev. S615-MM) as female and NIL of Gc1a (abbrev. CS-Gc1aGc1a). S615-MM carries four dominant marker genes; B1 (awn suppression, chromosome arm SAL), C (compact spikes, 2DL), Hg (hairy glumes, 1AL), and Hp1 (hairy peduncles, 4BL), despite all of the marker genes are recessive in CS. Speltoid suppressor Q is located on 5AL in both 'S-615' and CS, and the hetero- and hemizygote (Qq or Q.) are easily distinguished from the homozygote (QQ). Of 281 F1 progeny resulting from a cross between S615-MM (female) and CS-Gc1aGc1 a, 24 F1 progeny carried a mutated character(s) in any one or two of the marker genes. All except one exhibited the mutated phenotypes in all spikes. By contrast, in the 112 F1 progeny of the reciprocal cross, only two showed mutation of Q. This study revealed two important features of Gc1a in mutation induction: (1) Gc1a from male gametes can mutate genes derived from the female gametes (that is, mutation occurs after fertilization); and (2) induction of mutations ceases before differentiation of the shoot primordium.
To investigate the effect of Gc1a on the induction of mutations in more detail, two strains were produced by recurrent backcrosses: CS-MM carrying three marker genes (C, Hg and Hp1) in a CS background, and CS-MM-Gc1aGc1a possessing the markers together with Gc1a. Using these strains, manifestation of the marker and Q characters were observed (Table 3). As a result, the following three additional features of Gc1a were revealed: (3) When Gc1a exists in a female gamete, it does not mutate the genes on the genome of the same side (ref. Cross no. 3 of Table 3). In other words, presence of Gc1a in male gamete is the requirement for mutation induction. (4) Gc1a in a male gamete can mutate the genes in the same genome (Cross no. 4) but the frequency is lower than that in the opposite (female) side (Cross no. 2). And (5) when Gc1a is present in the female, the level of mutations induced by male Gc1a is reduced (Cross no. 6). It seems that the two Q mutants in Cross no. 6 were generated in the male gametes with Gc1a. The three mutants of C and probably three mutants of Q in the Cross no. 5 are thus highly likely to be mutated during male gametogenesis. In conclusion, the mode of inheritance of mutation appears to be similar to the mode observed for shriveled seeds. In the same way, chromosome 4Sl of Ae. sharonensis causes mutations at a higher frequency than Gc1a (Cross no. 7). However, chromosome 3C of Ae. triuncialis never caused mutation in zygotic cells (Cross no. 8). In order to test if the mutation is attributable to chromosome deletion or structural change of the marker genes, Tsujimoto and Noda (1989) selected many speltoid mutants of Q that arose independently in the offspring of Gc1a and/or Gc1b carriers and observed the chromosomes. All 22 of the mutants had deletions in the long arm of chromosome 5A, where Q is located.
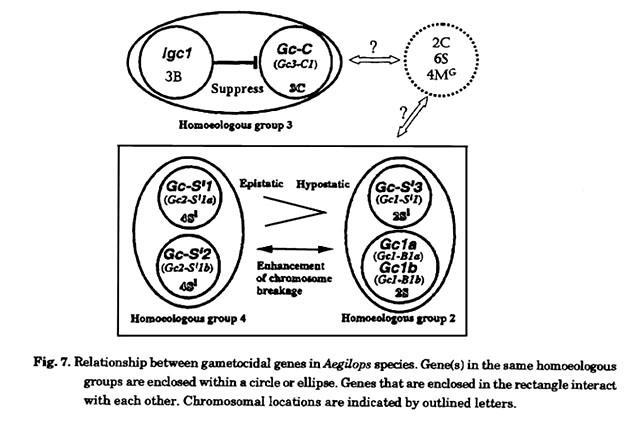
Interaction between gametocidal genes
By observing double monosomic addition lines derived from three different Gc chromosomes,Endo (1982) found that the Gc gene of Ae. triuncialis does not interact with the Gc gene of Ae. longissima or Ae. sharonensis. In addition, he found that the Gc gene of Ae. longissima dominated over the action of Ae. sharonensis, since only the Ae. longissima chromosomewas necessary for the gametes to function in the double monosoinic addition lines. Endo(1985) further reported that Gc genes located on chromosome 4Sl of Ae. longissima or Ae. sharonensis are epistatic to those on chromosome 2Sl, irrespective of species. Tsujimoto (1995) investigated the functional relationship among six Gc genes using plants with two different Gc genes (Fig. 7). From the study, it was concluded that there are three functional groups. The first group includes Gc genes located on the chromosomes belonging to the homoeologous group 2. Gc1a and Gc1b transferred to chromosome 2B of common wheat showed similar function to those on chromosome 2Sl of Ae. sharonensis. The second group includes the Gc genes on the chromosome 4Sl of Ae. sharonensis and Ae. longissima. These genes were epistatic to the Gc genes in the first group in terms of gamete abortion and preferential transmission, as indicated by Endo (1985). Although by themselves, the Gc genes in the first group cause chromosome breakage only at a low frequency (Tsujimoto and Tsunewaki 1985b; Tsujimoto and Noda 1989), these genes highly enhance breakage by Gc genes in the second group. Conversely, the Gc genes in the second group may enhance breakage by those in the first group.
The third group includes the Gc gene on chromosome 3C of Ae. triuncialis and is independent on the action of the Gc genes in the first or second group. The function of this Gc gene is suppressed by Igc1 on chromosome 3B of some common wheat strains. Based on the interactions between the different Gc genes, Tsujimoto (1995) proposed re-designation of the gene symbols following the rules for gene symbolization in wheat (McIntosh 1988). Tsujimoto (1995) proposed the name Gc1 for the Gc genes in the first group, Gc2 for the Gc genes in the second group, and Gc3 for the Gc genes in the third group, with each designation followed by the genome carrying the gene. The relationship between these Gc genes and those on chromosome 2C of Ae cylindrica; chromosome 4Mg of Ae. geniculata; and chromosome 6S of Ae. speltoides has not yet been examined.
Possible mechanism of chromosome breakage by Gc genes
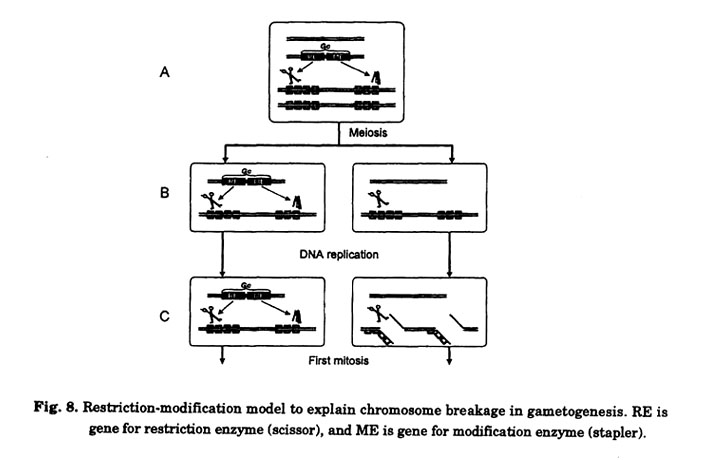
Tsujimoto and Tsunewaki (1985b) made note of the fact that the phenomena associated with Gc genes in wheat are similar to those observed for hybrid dysgenesis in the fruit fly, Drosophila. These observations include sterility, lethality, mutation, chromosome breakage, male recombination, and segregation distortion, and appeared only in the F1 progeny of a cross between P or I strains of Drosophila males and M or R strain females (Crow 1983; Bregliano and Kidwell 1983). Later, Tsujirnoto and Noda (1989) made note of the similarity between the nature of Gc genes and the restriction-modification systems found in many bacteria. In bacteria, a restriction endonuclease in the host cuts alien DNA at or around a particular base sequence. The host DNA, by contrast, is protected from digestion by methylation. This restriction-modification system provides a mechanism that could explain chromosome breakage in gametogenesis and in zygotic cells in wheat (Figs. 8 and 9). Here, we propose a model for Gc action in which a Gc gene is transferred to the wheat genome as Gc1a. The Gc gene produces both a restriction enzyme (RE) and a modification enzyme (ME) like a methylase. The RE cleaves the specific restriction sites that it recognizes (indicated by scissor in the Figs. 8 and 9). However, if the sites are protected by DNA methylation by the ME (indicated by stapler in Figs. 8 and 9), the RE cannot cleave (Fig. 8A). This would be the case in homozygotes for the Gc gene, where no chromosome breakage (indicated by scissor in the Figs. 8 and 9). However, if the sites are protected by DNA methylation by the ME (indicated by stapler in Figs. 8 and 9), the RE cannot cleave (Fig. 8A). This would be the case in homozygotes for the Gc gene, where no chromosome breakage appears. If ME function is incomplete and cannot protect all of the restriction sites (which would be likely soon after DNA replication, for example), chromosome breakage may appear at some frequency. After meiosis of the hemizygote of Gc gene, haploid cells without the Gc gene are generated (Fig. 8B). Prior to the first mitotic division in the gametogenesis, DNA is replicated. Since these cells would lack the ME, restriction sites on one of the strands of the replicated DNA are not modified. If the RE remains in the cell longer than the ME, or if RE can be supplied by other cells (for example, mother cells), the unmodified restriction sites are broken by the RE (Fig. 8C). In the following mitosis, unmodified DNA is broken in the same manner. Thus, the gametes without Gc become abortive. In this model the hemi-modified or hemi-methylated DNA must be deduced to cut by RE because chromosome breakage is observed in the first pollen mitosis.
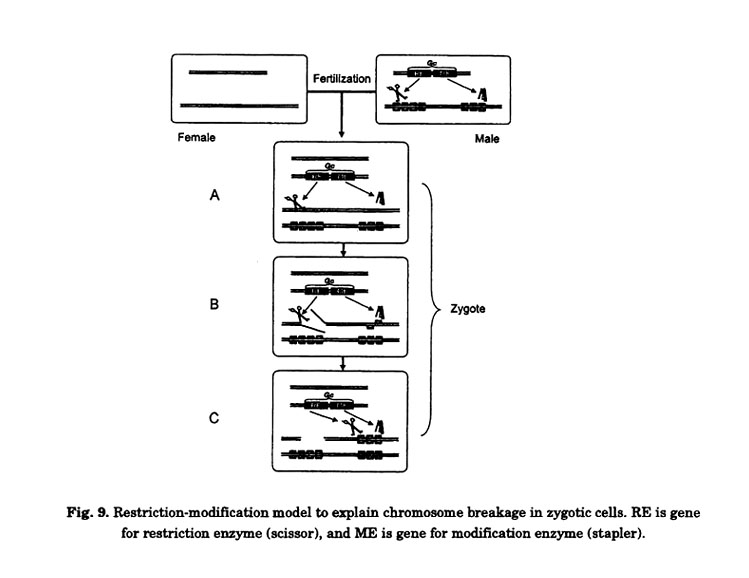
This model can also be used to explain chromosome breakage in zygotic cells, as outlined in Fig. 9. When pollen carrying the Gc gene fertilizes an egg cell without the Gc gene, unmodified DNA in the egg is exposed to RE from pollen (Fig. 9A). As a result, chromosomes are broken (Fig. 9B). However, the ME soon modifies the DNA derived from the egg and protects against RE (Fig. 9C). Thus, chromosome breakage ceases soon after fertilization.
de las Haras et al. (2001) observed that treatment of plants carrying the Gc gene of Ae. sharonensis with the hypomethylation agent 5-azacytidine induced chromosome breakage in root tip cells. The result suggested that the process of chromosome breakage in early seed development was repressed by DNA methylation. Friebe et al. (2003) isolated a mutant and chromosome breakage in hemizygous (Gc2mut/-)or heterozygous (Gc2mut/Gc2) conditions. The result clearly indicated that Gc2 encoded two agents that behave like the above-mentioned RE and ME for chromosome breakage and DNA protection, respectively. Because Gc2mut lost the function of the RE, Gc2mut/- plants did not show semi-sterility or chromosome breakage; and in addition, because Gc2mut retained ME function, Gc2mut/Gc2 plants were fertile and did not show induction of chromosome breakage.
The function of Gc2mut is similar to the function of suppressors of Gc genes. Indeed, if a mutant allele like as Gc2mut existed in the natural variation of wheat and was isolated, it would show the characteristics of a suppressor of Gc. Many Chinese and Japanese wheat cultivars, including N26, carry a dominant suppressor (Igc1) of the Gc gene on chromosome 3C from Ae. triuncialis. Both Igc1 and the Gc gene are located on the same homoeologous group, and further, Igc1 showed slight preferential transmission. This suppressor, therefore, may have the same function as the ME of the Gc gene in Ae. triuncialis. Tsujimoto and Tsunewaki (1985a) showed that about half of the female euploid gametes of the F1 progeny that result from a cross between N26 and CS+3C3C escaped from chromosome breakage. This result suggests that the escaped gametes carried Igc1 (ref. Fig. 4). At some point in the evolutionary past, wheat may have had a Gc gene on chromosome 3B that had a function similar to that of the Gc gene on chromosome 3C of Ae. triuncialis.
References
Bregliano JC and Kidwell MG (1983) Hybrid dysgenesis determinants. In: Shapiro JA (ed.) Mobile Genetic Elements. Academic Press, New York, pp. 363-410.
Crow JF (1983) Hybrid dysgenesis and P factor in Drosophila. Jpn J Genet 58:621-625.
de las Haras JI, King IP and Parker JS (2001) 5-azacytidine induces chromosomal breakage in root tips of wheat carrying the cuckoo chromosome 4SL from Aegilops sharonensis. Heredity 87:474479.
Endo TR (1978) On the Aegilops chromosomes having gametocidal action on common wheat. Proc V lnt Wheat Genet Symp, New Delhi 1: 306-314.
Endo TR (1979) Selective gametocidal action of a chromosome of Aegilops cylindrica in a cultivar of common wheat. Wheat lnf Serv 50:24-28.
Endo TR (1982) Gametocidal chromosomes of three Aegilops species in common wheat. Can J Genet Cytol 24:201-206.
Endo TR (1985) Two types of gametocidal chromosome of Ae. sharonensis and Ae. longissima. Jpn JGenet 60:125-135.
Endo TR (1988a) Induction of chromosome structural changes by a chromosome of Aegilops cylindrica L. in common wheat. J Hered 79:366-370.
Endo TR (1988b) Chromosome mutations induced by gametocidal chromosomes in common wheat. Proc VII Tnt Wheat Genet Symp, Cambridge 1: 259-263.
Endo TR (1990) Gametocidal chromosomes and their induction of chromosome mutation in wheat. Jpn J Genet 65:135-152.Endo TR and Gill BS (1996) The deletion stocks of common wheat. J Hered 87-295-307.
Endo TR and Tsunewaki K (1975) Sterility of common wheat with Aegilops triuncialis cytoplasm. J Hered 66:13-18.
Feldman M (1979) New evidence on the origin of -the B genome of wheat. Proc V Int Wheat Genet Symp, New Delhi 1:120-132.
Finch RA, Miller TE and Bennett MD (1984) "Cuckoo" Aegilops addition chromosome in wheat ensures its transmission by causing chromosome breaks in meiospores lacking it. Chromosoma 90:84-88.
Friebe B, Schubert V, Bluthner WD and Hammer K (1992) C-banding pattern and polymorphism of Aegilops caudata and chromosomal constitutions of the amphiploid T. aestivum - Ae. caudata and six derived chromosome addition lines. Theor Appl Genet 83: 589-596.
Friebe B, Kynast RG and Gill BS (2000) Gametocidal factor-induced structural rearrangement in rye chromosomes added to common wheat. Chromosome Res 8:101-511.
Friebe B, Kynast RG, Zhang P, Qi L, Dhar M and Gill BS (2001) Chromosome healing by addition of telomeric repeats in wheat occurs during the first mitotic divisions of the sporophyte and is a gradual process. Chromosome Res 9:137-146.
Friebe B, Zhang P, Nasuda S and Gill BS (2003) Characterization of a knock-out mutation at the Gc2 locus in wheat. Chromosoma 111:509-517.
Kihara H (1959) Fertility and morphological variation in the substitution and restoration backcrosses of the hybrids, Triticum vulgare x Aegilops caudata. Proc X Int Cong Genet, Montreal 1:142-171.
King IP and Laurie DA (1993) Chromosome damage in early embryo and endosperm development in crosses involving the preferentially transmitted 4SLl chromosome of Aegilops sharonensis. Heredity 70:52-59.
Kota RS and Dvorak J (1988) Genomic instability in wheat induced by chromosome 6Bs of Triticum speltoides. Genetics 120:1085-1094.
Kynast RG, Friebe B and GM BS (2000) Fate of multicentric and ring chromosome induced by a new gametocidal factor located on chromosome 4Mg of Aegilops geniculata. Chromosome Res 8:133139.
Maan SS (1975) Exclusive preferential transmission of an alien chromosome in common wheat. Crop Sci. 15:287-292.
Mcintosh RA (1988) Catalogue of gene symbols for wheat. Proc VII Int Wheat Genet Symp, Cambridge 2:1225-1323.
Miller TE (1983) Preferential transmission of alien chromosome in wheat. In: Brandham PE, Bennett MD (eds.) Kew chromosome conference II. George Allen & Unwin, London, pp. 173-182.
Nasuda S, Friebe B and Gill BS (1998) Gametocidal genes induce chromosome breakage in the interphase prior to the first mitotic cell division of the male gametophyte in wheat. Genetics 49: 1115-1124.
Nasuda S, Kikkawa Y, Ashida T, Rafiqul Islam AIM, Sato K and Endo TR (2005) Chromosomal allocation and deletion mapping of barley ESTs. Genes Genet Systems (in press).
Shi F and Endo TR (2000) Genetic induction of chromosome rearrangements in barley chromosome 7H added to common wheat. Chromosoma 109:358-363.
Qi LL, Echalier B, Chao S, Lazo GR, Butler GE, Anderson OD, Akhunov ED, Dvorak J, Linkiewicz AM, Ratnasiri A, Dubcovsky J, Bermudez-Kandianis CE, Greene RA, Kantety R, La Rota CM, Munkvold JD, Sorrells SF, Sorrells ME, Dilbirligi M, Sidhu D, Erayman M, Randhawa HS, Sandhu D, Bondareva SN, Gill KS, Mabmoud AA, Ma XF, Miftahudin, Gustafson JP, Conley EJ, Nduati V, Gonzalez-Hernandez JL, Anderson JA, Peng JH, Lapitan NL, Hossain KG, Kalavacharla V, Kianian SF, Pathan MS, Zhang DS, Nguyen HT, Choi DW, Fenton RD, Close TJ, McGuire PE, Qualset CO and Gill BS (2004) A chromosome bin map of 16,000 expressed sequence tag loci and distribution of genes among the three genomes of polyploid wheat. Genetics 168:701-12.
Tsujimoto H (1993) Molecular cytological evidence for gradual telomere synthesis at the broken chromosome ends in wheat J Plant Res 106:239-244.
Tsujimoto H (1994) Two new sources of gametocidal genes from Aegilops longissima and Ae. sharonensis. Wheat Inf Serv 79:42-46.
Tsujimoto H (1995) Gametocidal genes in wheat and its relatives. W. Functional relationship between six gametocidal genes. Genome 38:283-286
Tsujimoto H and Noda K (1989) Structure of chromosome 5A of wheat speltoid mutants induced by the gametocidal genes of Aegilops speltoides. Genome 32:1085-1090.
Tsujimoto H and Noda K (1990) Mutation of five marker genes in wheat by gametocidal gene of Ae. speltoides, Gc1a. Wheat Inf Serv 71:6-9.
Tsujimoto H and Tsunewaki K(1983) Genetic analyses on a gametocidal gene originated from Aegilops aucheri. Proc VI Int Wheat Genet Symp, Kyoto: 1077-1081.
Tsujimoto H and Tsunewaki K(1984) Gametocidal genes in wheat and its relatives. I. Genetic analyses in common wheat of a gametocidal gene derived from Aegilops speltoides. Can J Genet Cytol 26:78-84.
Tsujimoto H and Tsunewaki K (1985a) Gametocidal gene in wheat and its relatives. II. Suppressor of the chromosome 3C gametocidal gene of Aegilops triuncialis. Can J Genet Cytol 27:178-185.
Tsujimoto H and Tsunewaki K (1985b) Hybrid dysgenesis in common wheat caused by gametocidal genes. Jpn J Genet 6:565-578.
Tsujimoto H and Tsunewaki K (1985c) Seed shriveling caused by a gametocidal gene, Ge1. Wheat Inf Serv 60:40.
Tsujimoto H and Tsunewaki K (1988) Gametocidal genes in wheat and its relatives. III. Chromosome location and effects of two Aegilops speltoides-derived gametocidal genes in common wheat. Genome 30:239-244.
Tsujimoto H, Tsujimoto A and Tanaka 1(1990) Abnormal pollen development in wheat lines carrying the gametocidal genes. Jpn J Breed 40 (suppl. 2): 396-397 (in Japanese).
Tsujimoto H, Yamada T and Sasakuma T (1997) Molecular structure of a wheat chromosome end healed after gametocidal-gene-induced breakage. Proc Natl Acad Sci USA 94:3140-3144.
Tsujimoto H, Yamada T and Sasakuma T (1998) Pedigree of common wheat in East Asia deduced from distribution of the gametocidal inhibitor gene (Igc1) and beta -amylase isozymes. Breed Science 48:287-291.
Tsujimoto H, Usami N, Hasegawa K, Yamada T, Nagaki K and Sasakuma
T (1999) De novo synthesis of telomere sequence at the healed breakpoints of
wheat deletion chromosomes. Mol Gen Genet 262:851-856.
Tsujimoto H, Yamada T, Hasegawa K, Usami N, Kojima T, Endo TR, Ogihara Y and
Sasakuma T (2001) Large scale selection of lines with deletions in chromosome
1B in wheat and applications for fine deletion mapping. Genome 44: 501-508
Yen C, Luo MC and Yang JL (1988) The origin of the Tibetan weedrace of hexaploid wheat, Chinese Spring, Chendu-gang-tou and other landraces of the White Wheat Complex from China. Proc VII Int Wheat Genet Symp, Cambridge 1:175-179.