Memorial Issue, Wheat Information Service No.100
D genome of wheat - 60 years on from Kihara, Sears and McFadden
F. C. Ogbonnaya1,2, G. M. Halloran3 and E. S. Lagudah4*
1Primary Industries Research Victoria (PIRVic), Department
of Primary Industries, Horsham Centre, Private Bag 260, Horsham, Victoria 3401,
Australia
2Molecularar Plant Breeding Cooperative Research Centre, Victorian AgriBiosciences
Center, 1 Park Drive, Bundoora, Victoria 3083, Australia
34 James Service Place, South Melbourne, Victoria 3205, Australia
4CSIRO Plant Industry, GPO Box 1600, Canberra ACT 2601, Australia
*Corresponding author: Evans S. Lagudah
CSIRO Plant Industry, GPO Box 1600, Canberra ACT 2601, Australia
E-mail address: evans.lagudah@csiro.au
Key words: Aegilops tauchii, synthetic hexaploids, D genome, diploid, polyploid
Introduction
For more than half a century the pioneering findings of Kihara (1944), McFadden and Sears (1944, 1946) in the identification of Aegilops tauschii (syn Ae. squarrosa, Triticum tauschii) as the progenitor of the D genome of hexaploid wheat (T. aestivum) has remained undisputed. Amongst the progenitor species of hexaploid wheat, the diploid D genome progenitor has the widest geographic distribution from Turkey through West Asia to China. This wide geographic distribution coupled with an overall greater genetic variation relative to corresponding homologous loci in the D genome of hexaploid wheat has been the premise for continued analysis and speculation over the lineage(s) of Ae. tauschii that was involved in hexaploid wheat synthesis.
The wide genetic variation in Ae. tauschii has been exploited by various groups around the world for wheat improvement (Gill et al. 1985; see review by Lagudah et al. 1993; Ogbonnaya et al. 2003). Of the three genomes of hexaploid wheat, the D genome shows the least differentiation relative to its progenitor and therefore the expected usage of the diploid D genome in comparative genomics analysis in cereals (Gu et al. 2003; Chantret et al. 2005). The availability of large DNA insert (BAC) libraries and progress with construction of physical contigs of the diploid D genome (Moullet et al. 1999, http:llwheat.pw.usda.gov/ PhysicalMapping/) provides structural building blocks in providing further insights into the wheat and Triticeae genomes.
This review summarises local and genome-wide variation in the diploid and polyploid D genome, the comparative analysis with some of the collinear regions with rice and what can be expected of renewed attempts at broadening genetic variation of wheat via derivatives of amphidiploids from Ae. tauschii and T. turgidum conv. durum.
Patterns of variation in the D genome
Piecing together botanical, geographic and genetic variants : Different expeditions over the years, notably those led by Kihara (Kihara et al. 1965), and subsequent missions by other teams documented in the reports by Halloran (1968), Yen et al. (1983) and Jaaska (1995) have ensured that all known geographic regions where Ae. tauschii are found have been sampled (Fig. 1). These have enriched the available gene pool of the D genome. Ae. tauschii consists of four morphological varieties, three of which ('anathera', ',meyeri', 'typica') belong to ssp. tauschii while ssp. strangulata (Eig) contains only the variety strangulata. In the comprehensive report detailed by Kihara et al. (1965) the description of Ae. tauschii variants followed the morphological characteristics and showed ssp. strangulata to be localised in SE Caspian Sea region in Iran (Fig. 1) and var. meyeri in the SW region of the Caspian sea. By contrast var. typica was found in all the regions sampled. Subsequent analysis of the entire collection of Ae. tauschii revealed the occurrence of ssp. strangulata in two separate geographical regions, Transcaucasia (Armenia, Azerbaijan) and SE Caspian sea in Iran (Jaaska 1995; see Fig. 1). Most of the analysis of the corresponding variants between the D genome of T. aestivum and diploid D (Dt) shows a closer fit with ssp. strangulata than ssp. tauschii. Because of the two disjoined geographic regions of ssp. strangulata there have been divergent views on the "birthplace" of T. aestivum (Tsunewaki 1966, 1968; Nakai 1979; Jaaska 1980; Nishikawa et al. 1980; Dvorak et al. 1998).
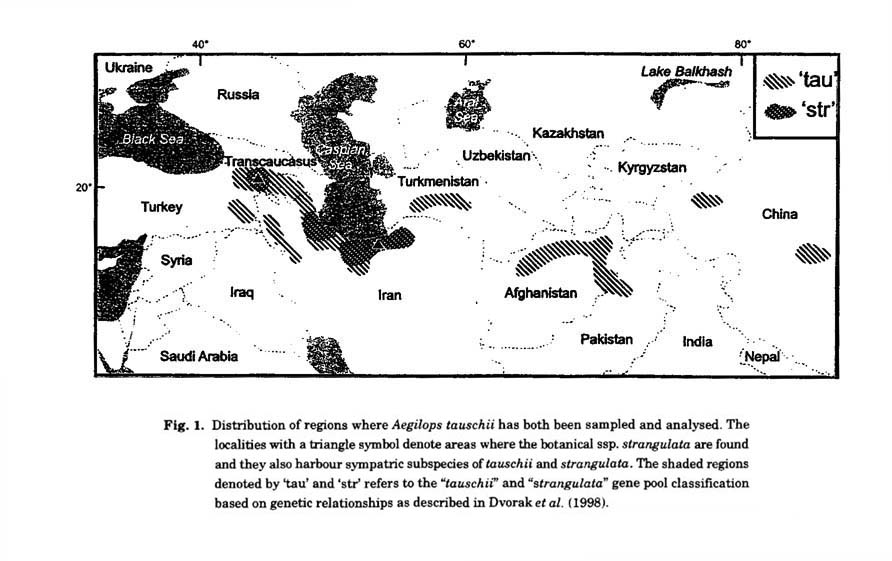
In using low copy DNA probes in restriction fragment length polymorphism (RFLP) analysis, Lubbers et al. (1991) and Tsunewaki et al. (1991) found the greatest variation in Iran and western Transcaucasia. The var. meyeri accessions identified by Kihara et al. (1965) as being localised in the SW Caspian sea was shown by Lubbers et al. (1991) to be much closer to ssp. strangulata than to the other varietal forms of ssp. tauschii. A comprehensive study undertaken by Dvorak et al. (1998) of representative samples from all regions known to harbour Ae. tauschii, concluded that the variation patterns were best illustrated by a gene-pool classification whereby the "strangulata gene pool" encompassed the var. meyeri and typica forms in SW Caspian as well as the classical botanical ssp. strangulata. Accordingly heterogeneity thus exists within the ssp. tauschii and involves populations that belong to both the "tauschii" and "strangulata" gene pools (Fig. 1). Dvorak et al. (1998) concluded that reference to the "strangulata" gene pool as the source of the D genome of T. aestivum rather than the botanical ssp. strangulata was a more correct description of the relationships between the respective D genomes. The gene pool classification lends further support to the findings of Lelley et al. (2000) who found a composite structure of genetic variants rather than an exclusiveness of ssp. strangulata as the major source of the D genome to hexaploid wheat.
Local and genome-wide variation : Several studies based on isozymes, storage proteins, simple and complex gene loci analysed via RFLP, simple sequence repeats (SSR) and single nucleotide polymorphism (SNP) haplotypes across the genome have shown an overall pattern in which for a given locus in the D genome of T. aestivum, a larger number of allelic variants exists in the diploid Dt genome. These observations lend support to the view that hexaploid wheat synthesis was limited to a few Ae. tauschii variants. In some instances variants have been identified in the D genome of T. aestivum where the corresponding Dt variant is non existent or yet to be identified.
Variation at the Gli-1 locus which encodes w- and g-gliadin storage proteins is highly polymorphic in Ae. tauschii and represents the top end of variant classes and approximates fingerprint haplotypes for a given genotype (Lagudah and Halloran 1988). How such large variation occurs at the Gli-1 locus in Dt population is not well understood. However, there is evidence for intra-locus recombination generating new variants (Metakovsky and Baboev 1992). Furthermore high recombination rates at the adjacent Glu-3 locus have been documented (Spielmeyer et al. 2000).
One of the well-characterised loci in Ae. tauschii and T. aestivum, at the gene and protein levels is the Glu-1 locus which encodes high-molecular-weight (HMW) glutenin subunits (Payne 1987; Payne and Lawrence 1983; Lagudah and Halloran 1988; Anderson et al. 1989, 2003). The Glu-1 locus comprises two genes and encodes paralogous x-type and y-type HMW subunits. Allelic variation at the Glu-D t1 locus from Ae.tauschii is higher than the corresponding Glu-D1 locus in T. aestivum. At least six alleles at the Glu-D1 locus have been identified although two of the alleles Glu-D1x2 +Dy12 and Glu-D1x5+ Dy10 are most prevalent. With the exception of the Glu-Dx5 gene, equivalent alleles have been identified at the Glu-Dt1 locus. While there are reports of Glu-Dtx5 subunits based on protein subunit mobility on SDS-PAGE gels, they differ from Glu-Dx5, which carries an extra cysteine residue (Pflueger et al. 2001). Thus the Glu-Dx5 gene is an example of a variant in the D genome that may have occurred subsequent to hexaploid synthesis or may be present in a rare Ae. tauschii accession yet to be identified. Similarly, of the two prevalent SNP haplotypes at the Gss locus encoding granule bound starch synthase present in the D genome across all T. aestivum subspecies, one of the variants was non-existent among a large and representative set of Ae. tauschii accessions (Caldwell et al. 2004).
An example of an extensively studied locus in the D genome of wheat where a monomorphic variant occurs is the Nor-D3 locus. The Nor-D3 locus that codes for the multi-gene ribosomal DNA shows a pattern of at least 15 non transcribed spacer variants in Ae. tauschii, whereas only a single variant, Nor-D3a which contains an array of seven 0.12 kb repeats, at the corresponding locus in T. aestivum (inclusive of ssp. aestivum, macha, spelta, vavilovii) has been identified to date (Appels and Dvorak 1982; Lassner et al. 1987; Lagudah et al. 1991b; Dvorak et al. 1998). The Nor-D3a variant was found primarily amongst accessions from ssp. strangulata.
Another class of genes that are increasingly being studied are disease resistance gene analogs (RGA) of the nucleotide binding site and leucine rich repeat (NBS-LRR) where variant forms occur at either simple or complex loci (Hulbert et al. 2001). The Vrga1 locus which encodes NBS-LRR members present in an Ae. ventricosa segment transferred to chromosome 2A (Seah et al. 2000) was studied at the equivalent homoeoloci, Vrga1-Dt present in Ae. tauschii. Analysis of a random set of accessions revealed that null haplotypes (or complete absence) of the Vrga1 family members were frequent among the accessions (Lagudah et al. 2001). Analyses of the D genome component present in Ae. ventricosa from a few samples have so far revealed the null haplotype; the extent of null haplotypes at the Vrga1-D locus in T. aestivum has not been determined. Because of the rapid changes associated with RGAs in plant genomes (Leister et al. 1998), this class of genes may be of limited use in comparative analysis of the Dt with other D genomes in polyploid wheat. However, where RGAS occur at, or are tightly linked to a disease phenotype they can be of predictive value in establishing allelic phenotypic variants derived from the D genome at the diploid and allohexaploid levels (Huang and Gill 2001).
The closest to a genome wide analysis of Ae. tauschii accessions was a study of 53 RFLP loci that covered both arms from all seven chromosomes (Dvorak et al. 1998). The average gene diversity was higher in the regions of Transcaucasia, SE Caspian, SW Caspian and North-Central Iran compared to areas in Turkey, Western Iran, Turkmenistan, Afghanistan and China. Differing levels of variation at the corresponding D genome of T. aestivum for the RFLP loci were found; in several loci a tendency towards the prevalence of two alleles occurred which parallels the observations made with the Gli-D1 g-gliadin (von Bueren 2001), Glu-D1, Gss and the ADP-glucose pyrophosphorylase loci (Caldwell et al. 2004). The frequency of the occurrence of two predominant variants in the T. aestivum D genome, may be indicative of a diphyletic lineage of the Dt genome involved in hexaploid wheat synthesis.
Insights into the D genome from large contiguous sequences
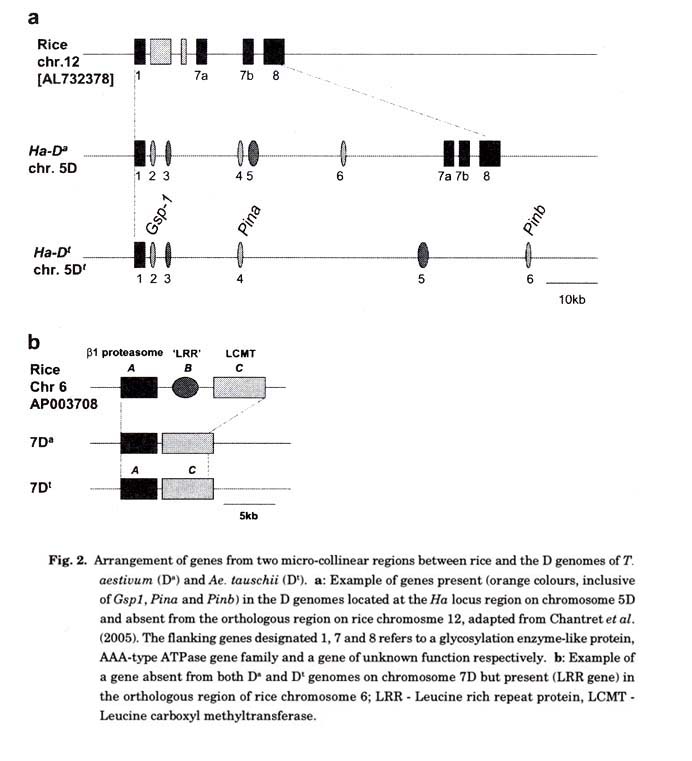
A progression from skeletal to saturated genetic maps (Kam-Morgan et al. 1989; Gill et al. 1991; Lagudah et al. 1991a; Boyko et al. 2002) and then to physical maps (http://wheat.pw.usda.gov/PhysicalMapping/ ) derived from large DNA insert libraries (BACs) and the partial sequences are yielding insights into the D genome of wheat. The genetic maps confirmed the conserved collinearity between the diploid and hexaploid D genomes. Contiguous sequences obtained from a few diploid D genome BACs, namely loci from Glu- Dt1 (Anderson et al. 2003), Ha (grain hardness/softness) (Chantret et al. 2005) and equivalent region of Lr21 (leaf rust resistance) (Brooks et al. 2002) have revealed a structure of gene islands within highly repetitive DNA. Comparison between the Ha locus region in the diploid and hexaploid D genomes showed a conserved gene order of the Gsp-1 (grain softness protein) and the puroindoline genes Pina and Pinb while the main differences were confined to the insertion of different retroelements (Chantret et al. 2005, see Fig. 2). Similar observations have been made between the Glu-D t1 and Glu-D1 locus, albeit with similar distances between the paralogous x and y gene members of 51 and 58 kb respectively (Yong Gu personal communication). Further comparisons between the D genome and orthologous regions in rice using the Ha locus region and partial sequences from BACs (derived from 7DtS and 7DS chromosome arms) containing beta1-proteasome subunits (Fig. 2) revealed examples of the "deletion" and "insertion" of genes in rice relative to the D genome in micro-collinear regions.
Structural integrity of D genome progenitor chromosomes relative to the D genome chromosomes of hexaploid wheat
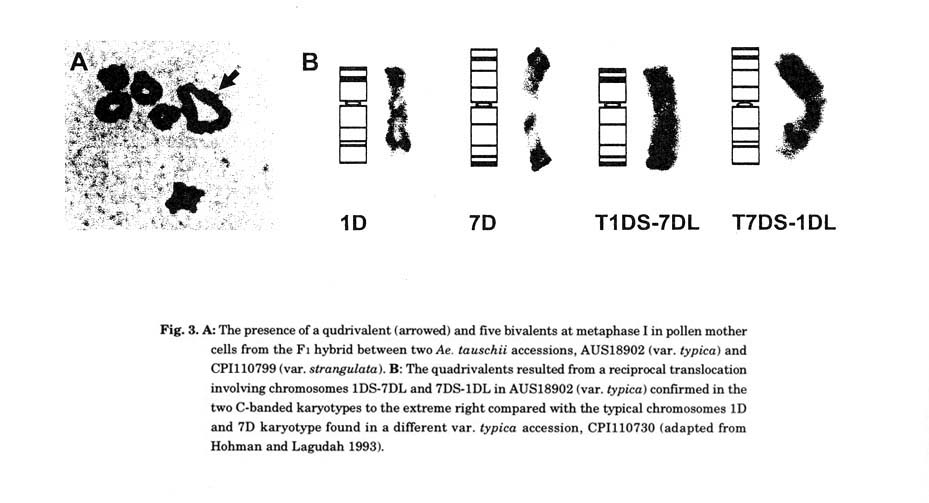
While the overall seven chromosomes of the Dt and D genomes T. aestivum are collinear, Kihara et al. (1965) noted an Ae. tauschii accession collected from Iran which gave rise to a ring of four chromosomes (quadrivalents) and five bivalents in crosses with other accessions. They concluded that a reciprocal chromosomal translocation was present in the Ae. tauschii accession. In later genetic mapping and cytogenetic studies by Lagudah et al. (1991b) and Hohman and Lagudah (1993), a pseudo linkage map that involved markers located on both the short and long arms of 1D and 7D in an Ae. tauschii hybrid were identified. From these studies unequivocal identification of a reciprocal translocation involving chromosomes 1DS-7DL and 7DS-1DL was established (Fig. 3). The occurrence of such translocations in the D genome of T. aestivum is yet to be documented. However, a reciprocal interchromosomal translocated wheat chromosome that involved the Lr34 gene (non-race specific adult plant rust resistance) on 7DS has been reported (Dyck et al. 1994).
Intra-specific differentiation in Ae. tauschii 7D chromosomes relative to its homolog in the D genome of T. aestivum was observed in metaphase pairing following the development of 7D substitution lines in the wheat cultivar, "Canthatch", using different morphological varieties of Ae. tauschii. While none of the substitution lines containing Ae. tauschii chromosomes had no major structural changes such as translocated segments, the chromosome 7D from the var. typica accession used showed reduced chromosome pairing than the corresponding chromosome from other Ae. tauschii varieties (Penner et al. 1986). Intraspecific divergence for at least chromosome 7D among Ae. tauschii accessions was suggested by the authors to have occurred prior to the synthesis of common hexaploid wheat. Polymorphic karyotype variants based on C-banding patterns of chromosomes 1D, 2D and 6D was used by Badaeva et al. (2002) to subdivide Ae. tauschii accessions into two groups. One group of the karyotypes was representative of ssp. strangulata and was similar to the D genome of common hexaploid wheat. The second group typified ssp. tauschii and were found to be similar to the D genome of other polyploid Aegilops species.
Differentiation in the D genome containing Aegilops polyploids
From the pioneering studies of Kihara (Kihara 1947, 1954, 1957, 1963; Kihara et al. 1959; Lilienfeld 1951) the phyletic relationships among the genomes of polyploid Aegilops species were defined and subsequently fine tuned by other researchers. Kihara's observations were founded on the prevalence of one pivotal genome homologous to a parental source while the other genomes were modified. Variations on the theme of genome modification were proposed by Zohary and Feldman (1962). Tetra- and hexaploid species founded on the pivotal D genome includes Ae. cylindrica (CcDc), Ae. ventricosa (DvNv), tetraploid Ae. crassa (XcrDcr1), hexaploid Ae. crassa (XcrDcr1Dcr2), Ae. juvenalis (Xj Dj Uj ) and Ae. vavilovii (Xva Dva Sva).
A combination of meiotic chromosome pairing (Zhao and Kimber 1984), isozyme analysis (Nakai 1982), DNA repeat sequence variants (Rayburn and Gill 1987; Dubcovsky and Dvorak 1994; Zhang and Dvorak 1992; McNeil et al. 1994) and various karyotype analysis (Badaeva et al. 2002), have identified varying levels of modification of the D genome. On one end of the spectrum are the D genomes of T. aestivum, Ae. cylindrica and hexaploid Ae. crassa (Dcr2 genome) with little modification relative to Ae. tauschii and on the other end with substantial modification are Ae. juvenalis and Ae. vavilovii. Badaeva et al. (2002) from studies using comparative C-banding and pAs1-FISH analysis found the Dc and Dcr2 genomes of Ae. cylindria and hexaploid Ae. crassa to be virtually identical to the ssp. tauschii genome, while the D genome of T. aestivum was similar to that of ssp. strangulata. McNeil et al. (1994) isolated a repeated sequence, Dgas44-3, from Ae. tauschii which was shown to contain D genome specific elements dispersed on all D genome chromosomes of T. aestivum. The "Dgas44-3" element was confirmed to be also amplified in the Dc, Dv, Dcr2 genomes but not in the Dj, Dva and Dcr1 of the polyploid Aegilops species. Given the wide gene pool of Ae. tauschii, it remains to be shown whether there are accessions that lack the amplified "Dgas44-3" element and therefore reveal a variant D genome that is more closely related to the Dj, Dva and Dcr1 found in Ae. juvenalis, Ae. vavilovii and tetraploid Ae. crassa.
Impact of the D genome in wheat improvement
Introgressing Dt variants into D genome of common hexaploid wheat : Ever since the first report by McFadden and Sears (1944) in the artificial synthesis of hexaploid wheat, numerous trait transfers aimed at testing expression levels in particular Dt variants at the allohexaploid level have been performed. Subsequent backcrosses into adapted bread wheat cultivars to generate 'synthetic derived backcrossed bread lines (SBLs)' are being pursued to develop novel recombinant genotypes to widen the existing primary gene pool of bread wheat. Such novel genotypes result from either new Dt or AdBd (from durum) derived alleles or interactions between these new variants and the bread wheat genome. Thus a measure of the impact of SBLs is not exclusive to the introgression of the Dt variants. Other approaches based on (i) direct crosses between Ae. tauschii and bread wheat followed by subsequent backcrosses (Gill and Raupp 1987) eliminates the confounding effects of new variants from the AdBd genome; (ii) development of specific Dt introgressed segments starting from Ae. tauschii single chromosome substitutions in bread wheat (Pestsova et al. 2002). The availability of SBLs and direct Dt introgressed bread wheat lines has extended the exploitation of the Ae. tauschii 'gene pool' beyond qualitative traits into quantitative characters such as yield and adaptability for wheat improvement.
Enhancing yield potential : Cox et al. (1995) demonstrated the possibility of yield increases in hexaploid wheat from Ae. tauschii backcrossed derived populations. The yield increases in some of the progenies were a result of changes in yield components and in particular, increases in 1000-grain weight relative to the recurrent parent. Similar observations were made in SBLs where higher yielding lines were associated with increased rates of grain .filling and larger 1000-grain weight, in particular in lower-yielding environments with drought stress (del Blanco et al. 2000; Gororo et al. 2002)
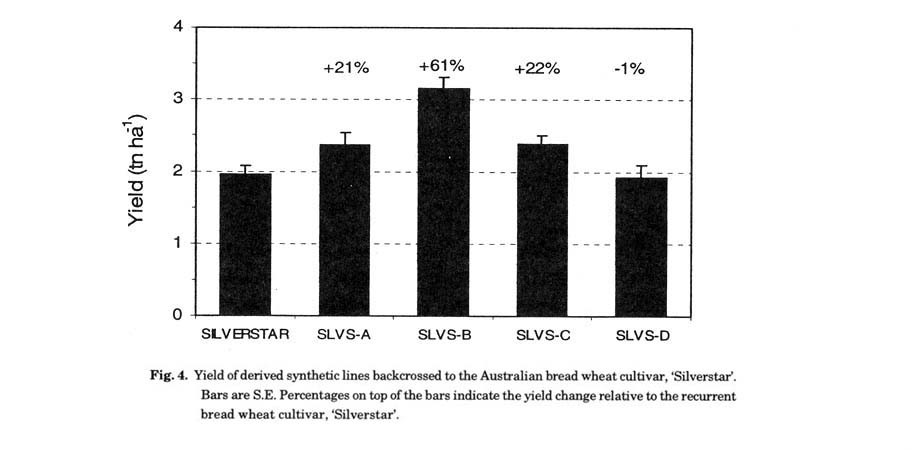
Ogbonnaya et al. (2003) reported the results of the evaluation of SBLs at CIMMYT under induced drought treatment where yield of the SBLs under drought was on average 10 to 41% higher than that of the Australian cultivars across trials, with substantial variation among lines (Fig. 4). The range in yield of the SBLs suggests considerable potential for selection of enhanced yield amongst these populations for moisture limiting environments. In a limited set of data based on multi-environmental trials in Australia across 5 sites, using the SBLs selected in Mexico, many of the SBLs yielded more than some of the locally adapted check cultivars depending on the environment. In general, there was more consistency in the behaviour of the SBLs in the Northern region of Australia, where crops are grown on stored moisture as in the Mexico selection environment (CIMMYT, Cd Obregon), than in other environments in Australia (Dreccer and Ogbonnaya unpublished). It remains to be shown whether the genomic regions contributing to the yield gains are derived from the Dt or AdBd variants. Furthermore the underlying physiological basis for yield gains under moisture limiting environments are yet to be defined. Some suggestions include increased capacity for water extraction during critical grain growth phase, more vigorous root systems and increased root density observed in SBLs relative to bread wheat cultivars (Dreccer et al. 2004a).
From the results of the CIMMYT, Mexico and Australia trials, some SBLs significantly outperformed the elite bread wheat parents in yield. These SBLs are an invaluable resource for the targeted search and identification of genes underlying the QTLs derived from Dt or AdBd genomes that are associated with agronomically-significant genetic variation for yield and yield components under limited moisture. In a recent study to identify introgressed regions in SBLs, SSR markers spread across the wheat genome were used to locate a small number of allelic variants inherited from the Dt or AdBd genomes with a selection advantage following several generations of selection (Zhang et al. 2005). As more genome wide approaches are adopted to analyse SBLs, it is anticipated that some answers to the following questions can be addressed: can the defined genomic regions from SBLs be used to estimate the effect and mode of action of the QTLs, including the interactions amongst the genetic factors controlling these traits? What measures of agronomic or physiological performance are associated with the defined genomic regions? Are the genomic regions with a selection advantage limited to a few, or occur in a broad range of recurrent elite bread wheat backgrounds? Some of the answers to these questions will impact on directed selection of Dt variants from the wider gene pool of Ae. tauschii for improving quantitative traits.
Grain, flour and dough quality: Functional differences in grain and flour quality between common hexaploid and tetraploid (T. turgidum ssp. durum) wheats have been attributed to the influence of the D genome. Thus the overall viscoelastic properties that confer bread making characteristics of common hexaploid wheat in contrast to the pasta making characteristics of durum wheat reflects the impact of the D genome. While various aspects of grain, flour and dough quality have been studied to further partition components of the D genome that exerts major differences, most of the studies have revealed the significant role of gluten and puroindoline proteins in flour functionality. Although variation at the Glu-Dt1 locus that controls HMW glutenins in Ae. tauschii is much higher than the corresponding locus in T. aestivum, none of the novel variants transferred into bread wheat from Ae. tauschii has so far revealed superior breadmaking characteristics to what already exists in bread wheat cultivars (Bennett 1994).
Variation at the Ha locus, the major determinant of grain 'hardness'or 'softness' has revealed complete deletions in the Pina and Pinb genes (Fig. 2) present at the A and B genomes of common wheat, and phenotypic differences in grain hardness/softness is largely due to the locus on the D genome resulting from variants deleted in either Pina and/or mutations in Pinb (Giroux and Morris 1998; Beecher et al. 2002; Turnbull et al. 2003; Chantret et al. 2005). With the additional variants reported in Pina and Pinb genes in Ae. tauschii, the scope for widening grain texture and how it may impact on end-use properties when introgressed into bread wheat is the subject of ongoing analysis (Gedye et al. 2004; Massa et al. 2004).
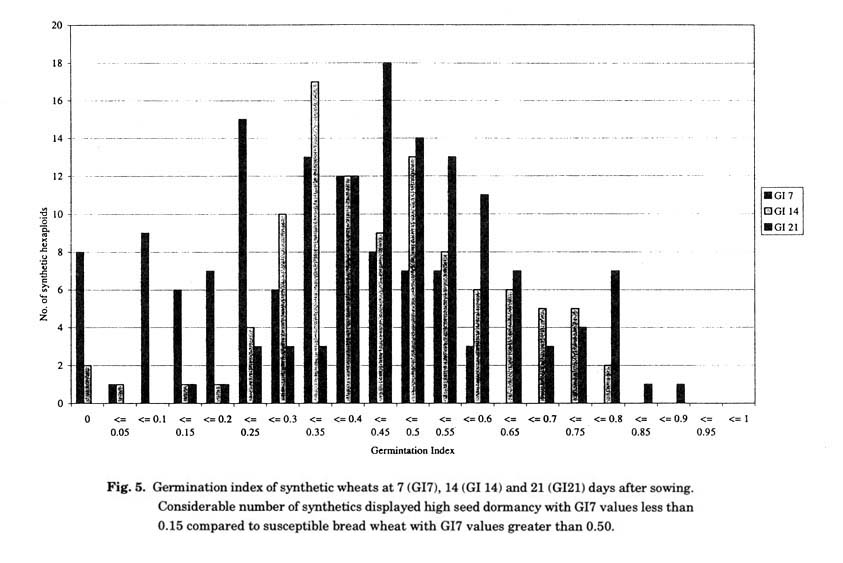
Pre-harvest grain sprouting: Despite several decades of research into resistance to pre harvest sprouting (PHS), its levels in most white-grained wheat cultivars are still inadequate. Seed dormancy, the temporary failure of a viable seed to germinate under favourable conditions, provides PHS resistance in cereal crops. Because of limited variation in sprouting resistance in hexaploid wheat, efforts are being directed towards the use of wild relatives of wheat including Ae. tauschii, which exhibit some measure of dormancy. A number of mechanisms have been identified for resistance to PHS in Ae. tauschii. One mechanism is a novel water-soluble inhibitor of germination in the bracts surrounding the grain (Gatford et al. 2002), while other potentially useful mechanisms are all grain borne. Ae tauschii exhibited variable levels of grain dormancy in which time to 50% germination ranged from 5 to 21 days (Gatford et al. 2002). Comparable levels of grain dormancy were observed amongst synthetic hexaploids, in which some did not germinate seven days after sowing (Fig. 5). The use of Ae. tauschii source of seed dormancy in conferring PHS resistance in hexaploid wheat has recently been demonstrated in SBLs (Hearnden, Imtiaz and Ogbonnaya unpublished).
Several QTLs associated with PHS resistance has been identified and include the major phs locus on 4AL as well as the triplicate loci controlling the red seedcoat colour, R gene, on the long arms of the group 3 chromosomes (Flintham et al. 2002). The D genome of hexaploid wheat possesses the fewest QTLs for PHS resistance of the three contributing genomes and efforts have been directed at exploring the varying levels of seed dormancy which is also expressed in synthetic hexaploid (Fig. 5). Recently, a novel QTL was identified on chromosome 1D that explained upto 55% of the phenotypic variance for seed dormancy in SBLs (Hearnden 2004). The availability of SBLs derived from different Ae. tauschii and synthetic hexaploids expressing high levels of seed dormancy offers the opportunity to investigate natural allelic variation at seed dormancy in wheat. The broad spectrum of seed dormancy exhibited in the Dt genome as a diploid relative to the hexaploid provides further evidence in support of a "genetic bottleneck" during the evolution of hexaploid wheat.
Disease resistance : Numerous examples in the identification of, and introgression of traits conferring resistance to fungal, viral, nematode and insect pests continues to reflect the principal area of activity in exploiting the wider variation found in the diploid D genome of wheat (see review Lagudah et al. 1993). In the case of the leaf rust resistance trait, Lr21, derived from Ae. tauschii and transferred into bread wheat, the gene has been cloned and shares features of the NBS-LRR disease resistance proteins (Huang et al. 2003). In studies of the Cre3 locus in Ae. tauschii which controls cereal cyst nematode (CCN) resistance, RGA sequences derived from the locus have proven to be valuable markers for selection and breeding of Cre3 and other CCN genes in bread wheat (Eastwood et at. 1994; Lagudah et al. 1997; Ogbonnaya et al. 2001; de Majnik et al. 2003).
Prospects for improving salt tolerance : Another trait where the D genome has impacted on the adaptation of common hexaploid wheat over durum wheat is its relative tolerance to salinity. Overall salinity tolerance of bread wheat in contrast to intolerant durum wheat is associated with a relatively high ability to exclude Na+ from the leaf blades and an overall increase in the K+/Na+ ratio, in some cases it is associated with an increase in K+ uptake (Gorham et al. 1987; Dvorak et al. 1994). A major locus, Kna1, controlling K+/Na+ uptake has been shown to be present on chromosome 4DL (Dubcovsky et al. 1996).
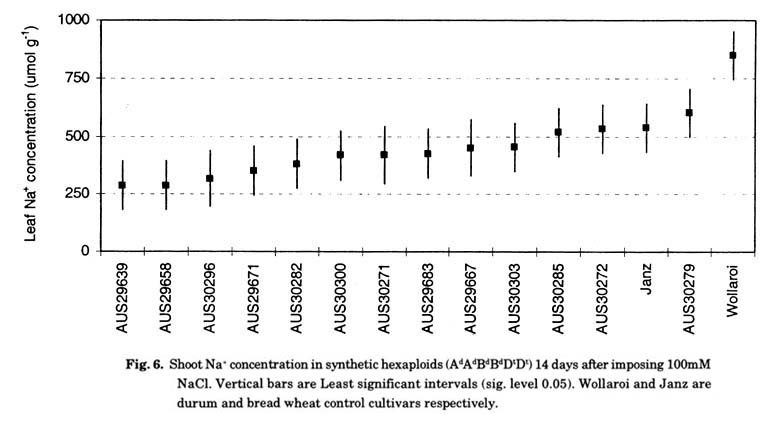
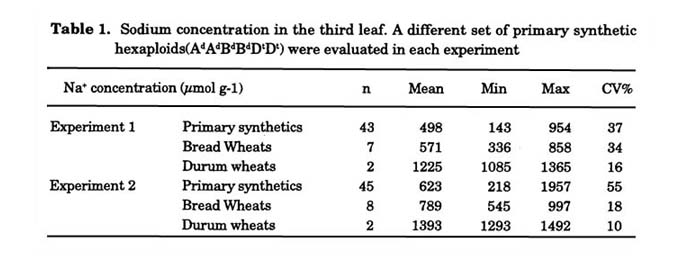
Substantial genetic variation for salt tolerance was previously reported in Ae. tauschii and has been shown to be expressed at the hexaploid level (Farooq et al. 1989; Schachtman et al. 1991, 1992; Prichard et al. 2002). summary of genetic variation in Na+ exclusion ability present in a set of primary synthetics is shown in Fig. 6 (Dreccer et al. 2004b). In one experiment, 17 primary synthetics had significantly lower Na+ concentration (P < 0.05) than the bread wheat cultivar with the highest Na+ concentration (Table 1). Consistently the durum wheats were more salt sensitive than bread wheat.
The Na+ concentration in the shoot at 100 mM NaCl (sheaths + leaves) tended to be lower in most of the primary synthetics compared to a reference bread wheat cultivar, Janz, though the differences were not significant. Genotypic differences in Na+ concentration in the shoot were closely linked to variation in the net uptake rate of Na+ (Dreccer et al. 2004b). The net uptake rate of Na+ also explained a significant proportion of the variation observed in the rate of Na+ transport to the shoot (data not shown), suggesting that additional mechanism maybe involved in salinity tolerance from Ae. tauschii .It remains to be shown whether the full spectrum of variation observed in either Ae. tauschii or the primary synthetics is due to the effect of the Kna1 locus. The identification of different mechanisms or loci independent of Kna1 could potentially contribute to enhance and diversify the basis of salt tolerance in current bread wheat cultivars.
References
Anderson OD, Greene FC, Yip RE, Halford NG, Shewry PR and Malpica-Romero JM (1989) Nucleotide sequences of the two high-molecular-weight glutenin genes from the D-genome of a hexaploid bread wheat, Triticum aestivum L. cv Cheyenne. Nucleic Acids Res 17: 461-462.
Anderson 0 D, Rausch C, Moullet 0 and Lagudah ES (2003) The wheat D genome HMW glutenin locus: BAC sequencing, gene distribution, and retrotransposon clusters. Fuction Integr Genomics 3: 56-68.
Appels R and Dvorak J (1982) The wheat robisomal DNA spacer: its structure and variation in populations and among species. Theor Appl Genet 63: 337-348.
Badaeva ED, Amosova AV, Muravenko OV, Samatadze TE, Chikida NN, Zelenin AV, Friebe B and Gill BS (2002) Genome differentiation in Aegilops. 3. Evolution of the D-genome cluster. Plant Syst Evol 231: 163-190.
Beecher B, Bettege A, Smidansky E and Giroux MJ (2002) Expression of wild-type pinB sequence in transgenic wheat complements a hard phenotype. Theor Appl Genet 105:870-877.
Bennett AM (1994) Molecular and functional studies of variant HAM glutenins in wheat PhD thesis, University of Sydney, Australia.
Boyko E, Kalendar R, Korzun V, Fellers J, Korol A, Schulman AH and Gill BS (2002) A high-density cytogenetic map of the Aegilops tauschii genome incorporating retrotransposons and defense-related genes: insights into cereal chromosome structure and function. Plant Mol Biol 48: 767 789.
Brooks SA, Huang L, Gill BS and Fellers JP (2002) Analysis of 106 kb of contiguous DNA sequence from the D genome of wheat reveals high gene density and a complex arrangement of genes related to disease resistance. Genome 45: 963-972.
Caldwell, KS, Dvorak J, Lagudah ES, Akhunov E, Luo MC, Wolters P and Powell W (2004) Sequence polymorphism in polyploid wheat and their D-genome diploid ancestor. Genetics 167: 941-947.
Chantret N, Salse J, Sabot F, Rahman S, Bellec A, Laubin B, Dubois I, Dossat C, Sourdille P, Joudrier P, Gautier MF, Cattolico L, Beckert M, Aubourg S, Weissenbach J, Caboche M, Bernard M, Leroy P and Chaloub B (2005). Molecular basis of evolutionary events that shaped the hardness locus in diploid and polyploid wheat species (Triticum and Aegilops). Plant Cell 17: 1033-1045.
Cox TS, Sears RG, Bequette RK and Martin TJ (1995) Germplasm enhancement in winter wheat x Triticum tauschii backcross populations. Crop Sci 35: 913-919.
del Blanco IA, Rajaram S. Kronstad WE and Reynolds MP (2000) Physiological performance of synthetic hexaploid wheat-derived populations. Crop Sci 40: 1257-1263.
de Majnik J, Ogbonnaya FC, Moullet 0 and Lagudah ES (2003) The Cre1 and Cre3 nematode resistance genes are located at homoeologous loci in the wheat genome. Mol Plant-Microbe Interact 16: 1129-1134.
Dreccer F, Ogbonnaya FC, Borgognone G and Wilson J (2004a) Variation in shoot and root growth in primary synthetics wheat - implications for overcoming water deficits in marginal environments. "New Dimensions for a diverse planet". Proc IV Int Crop Sci Congr, Brisbane. Published on CDROM. Web site www.regional.org.au/au/cs.
Dreccer MF, Ogbonnaya FC and Borgognone G (2004b) Sodium exclusion in primary synthetic wheats. Proc XI Wheat Breeding Assembly: 118-121.
Dubcovsky J and Dvorak J (1995) Genome origin of Triticum cylindricum, Triticum triunciale, and Triticum ventricosum (Poaceae) inferred from variation in repeated nucleotide sequences: A methodological study. Amer J Bot 81: 1327-1335.
Dubcovsky J, Sant Maria G, Epstein E, Luo MC and Dvorak J (1996) Mapping of the K+/Na+ discrimination locus Kna1 in wheat. Theor Appl Genet 92: 448-454.
Dvorak J, Luo MC, Yang ZL and Zhang HB (1998) The structure of the Aegilops tauchii genepool and the evolution of hexaploid wheat. Theor Appl Genet 97: 657-670.
Dvorak J, Noaman MM, Goyal S and Gorham J (1994) Enhancement of the salt tolerance of Triticum turgidium L. by the Kna1 locus transferred from Triticum aestivum L. chromsome 4D by homoeologous recombination. Theor Appl Genet 87:872-877.
Dyck PL, Kerber ER and Aung T (1994) An interchromosomal reciprocal translocation in wheat involving leaf rust resistance gene Lr34. Genome 37: 556-559.
Eastwood RF, Lagudah ES and Appels R (1994) A directed search for DNA sequences tightly linked to cereal cyst nematode resistance genes in Triticum tauschii. Genome 37: 311-319.
Farooq S, Naizi MLK, Iqbal N and Shah TM (1989) Salt tolerance potential of wild resources of the tribe Triticeae: II. Screening of species of the genus Aegilops. Plant Soil 119: 255-260.
Flintham J, Adam R, Bassoi M, Holdsworth M and Gale M (2002) Mapping genes for resistance to sprouting damage in wheat. Euphytica 126: 39-45.
Gatford KT, Hearuden P, Ogbonnaya F, Eastwood RF and Halloran GM (2002) Novel resistance to pre-harvest sprouting in Australian wheat from the wild relative Triticum tauschii. Euphytica 126: 67-76.
Gedye KR, Morris CF and Bettge AD (2004) Determination and evaluation of the sequence and textural effects of the puro-indoline a and puro-indoline b genes in a population of synthetic hexaploid wheat. Theor Appl Genet 109: 1597-1603.
Gill BS and Raupp WJ (1987) Direct genetic transfers from Aegilops squarrosa L. to hexaploid wheat. Crop Sci 27: 445-450.
Gill BS, Sharma HC, Raupp WJ, Browder LE, Hatchett JH, Harvey TL, Moseman JG and Waines JG (1985) Evaluation of Aegilops species for resistance to wheat powdery mildew, wheat leaf rust, hessian fly, and greenbug. Plant Disease 69: 314-316.
Gill KS, Lubbers EL, Gill BS, Raupp WJ and Cox TS (1991) A genetic linkage map of Triticum tauschii (DD) and its relationship to the D genome of bread wheat (AABBDD). Genome 34: 362-374.
Giroux MJ and Morris CF (1998) Wheat grain hardness results from highly conserved mutations in the friabilin components Puro-indoline a and b. Proc Natl Acad Sci USA 95:6262-6266.
Gorham J, Hardy C, Wyn-Jones RG, Joppa LR and Law CN (1987) Chromosomal location for a Ka:Na discrimination character in the D genome of wheat. Theor Appl Genet 74: 584-588.
Gororo NN, Eagles HA, Eastwood RF, Nicolas ME and Flood RG (2002) Use of Triticum tauschii to improve yield of wheat in low-yielding environments. Euphytica 123:241-254.
Gu YQ, Anderson OD, Londeore CF, Kong X, Chibbar RN and Lazo G (2003) Structrural organisation of the barley D-hordein locus in comparison with its orthologous regions of wheat genomes. Genome 46: 1084-1097.
Halloran GM (1968) Wheat collecting expedition to Afghanistan. In: Finlay KW and K.W. Shepherd KW (eds.) Proc III Int Wheat Genet Symp, Canberra, pp. 159-160.
Hearnden P (2004) A molecular genetic study of seed dormancy in Aegilops tauschii and expression of sprouting resistance in common hexaploid wheat. PhD thesis, Institute of Land and Food Resources, University of Melbourne.
Hohman U and Lagudah ES (1993) C-banding polymorphism and linkage of non-homologous RFLP loci in the D genome progenitor of wheat. Genome 36: 235-243.
Huang L and Gill BS (2001) An RGA-like marker detects all known Lr21 leaf rust resistance gene family members in Aegilops tauschii and wheat. Theor Appl Genet 103: 1007-1013.
Huang L, Brooks SA, Li WL, Fellers JP, Trick HN and Gill BS (2003) Map-based cloning of leaf rust resistance gene Lr21 from the large and polyploidy genome of bread wheat. Genetics 164: 655 664.
Hulbert SH, Webb CA, Smith SM and Sun Q (2001) Resistance gene complexes: Evolution and utilization. Ann Rev Phytopath 39: 285-312.
Jaaska V (1980) Electrophoretic survey of seedling esterases in wheats in relation to their phylogeny.
Theor Appl Genet 56: 273.284.
Jaaska V (1995) Isoenzymes in the evaluation of germplasm diversity in wild diploid relatives of cultivated wheat. In: Damania AB (ed) Biodiversity and Wheat Improvement. ICARDA, Wiley-Sayce Publ, pp 247-257.
Kam-Morgan LNW, Gill BS and Muthukrishnan S (1989) DNA restriction fragment length polymorphisms: a strategy for genetic mapping of D genome of wheat. Genome 32: 724-732.
Kihara H (1944) Discovery of the DD analyser, one of the ancestors of Triticum vulgare (in Japanese). Agri Horti 19: 889-890.
Kihara H (1947) The genus Aegilops classified on the basis of genome analysis. Seiken Ziho 3: 7-25.
Kihara H (1954) Considerations on the evolution and distribution of Aegilops species based on the analyser-method. Cytologia 19:336-357.
Kihara H (1957) Completion of genome-analysis of three 6x species of Aegilops. Wheat Inf Serv 6: 11.
Kihara H (1963) Interspecific relationship in Triticum and Aegilops. Seiken Ziho 15:1-12.
Kihara H, Yamashita K and Tanaka M (1965) Morphological, physiological, geographical and cytological studies in Aegilops and Triticum collected in Pakistan, Afghanistan and Iran. In: Yamashita K (ed.) Cultivated Plants and their Relatives, Koei Printing, Japan, pp. 1-118.
Lagudah ES and Halloran GM (1988) Phylogenetic relationships of Triticum tauschii the D genome donor to hexaploid wheat. 1. Variation in HAM subunits of glutenin and gliadins. Theor Appl Genet 75: 592-598.
Lagudah ES, Appels R, Brown AHD and McNeil D (1991a) The molecular-genetic analysis of Triticum tauschii - the D genome donor to hexaploid wheat. Genome 34: 375-386.
Lagudah ES, Appels R, Brown AHD and McNeil D (1991b) The Nor-D3 locus of Triticum tauschii: natural variation and linkage to markers in chromosome 5. Genome 34:387-395.
Lagudah ES, Appels R, McNeil D and Schachtman DP (1993) Exploiting the diploid D genome chromatin for wheat improvement. In: Gustafson JP, Appels R and Raven P (eds.) Gene Conservation and Exploitation. Plenum Press, New York, pp. 87-107.
Lagudah ES, Dubcovsky J and Powell W (2001) Wheat genomics. Plant Physiol Biochem 39: 335- 344.
Lagudah ES, Moullet 0 and Appels R (1997) Map based cloning of a gene sequence encoding a nucleotide binding domain and leucine rich region at the Cre3 nematode resistance locus of wheat. Genome 40: 659-665.
Lassner M, Anderson 0 and Dvorak J (1987) Hypervariation associated with a 12-nucleotide direct repeat and inferences on intragenomic homogenization of robisomal RNA gene spacer based on the DNA sequence of a clone from the wheat Nor-D3 locus. Genome 29: 770-781.
Leister D, Kurth J, Laurie DA, Yano M, Sasaki T, Devos K, Graner A and Schuize-Lefert P (1998) Rapid reorganization of resistance gene homologues in cereal genomes. Proc Natl Acad Sci USA 95: 370-375.
Lelley T, Stachel M, Grausgruber H and Vollmann J (2000) Analysis of relationships between Aegilops tauschii and the D genome of wheat utilizing microsatellites. Genome 43: 661-668.
Lilienfeld FA (1951) H. Kihara: Genome-analysis in Triticum and Aegilops. X. Concluding review. Cytologia 16: 101-123.
Lubbers EL, Gill KS, Cox TS and Gill BS (1991) Variation of molecular markers among geographically diverse accessions of Triticum tauschii. Genome 34: 354-361.
Massa AN, Morris CF and Gill BS (2004) Sequence diversity of puroindoline-a, puroindoline-b, and the grain softness protein genes in Aegilops tauschii Coss. Crop Sci 44: 1808-1816.
McFadden ES and Sears ER (1944) The artificial synthesis of Triticum spelta. Rec Genet Soc Am 13: 26-27.
McFadden ES and Sears ER (1946) The origin of Triticum spelta and its free-threshing hexaploid relatives. J Hered 37: 81-89.
McNeil D, Lagudah ES, Hohmann U and Appels R (1994) Amplification of DNA sequences in wheat and its relatives: the Dgas44 and R350 families of repetitive sequences. Genome 37: 320-327.
Metakovsky EV and Baboev SK (1992) Polymorphism and inheritance of gliadin polypeptides in Triticum monococcum L. Theor Appl Genet 84:971-978.
Moullet 0, Zhang HB and Lagudah ES (1999) Construction and characterisation of a large DNA insert library from the D genome of wheat. Theor Appl Genet 99: 305-313.
Nakai Y (1979) Isozyme variations in Aegilops and Triticum. IV. The origin of common wheats revealed from the study on esterase isozymes in synthesized hexaploid wheats. Japan J Genet 54: 175 189.
Nakai Y (1982) D genome donors for Aegilops crassa (DDMM, DDDDMM) and Ae vavilovii (DDMMSS) deduced from esterase analysis by isoelectric focusing. Japan J Genet 57: 349-360.
Nishikawa K, Furuta Y and Wada T (1980) Genetic studies on a-amylase isozymes in wheat. III. Intraspecific variation in Aegilops squarrosa and birthplace of hexaploid wheat. Japan J Genet 55: 325-336.
Ogbonnaya FC, Dreccer F, Trethowan R, Winter B, Eastwood R, Sheppard J and Lagudah ES (2003) Exploitation of synthetic wheats for agronomically useful genes - Current status. Proc X Int Wheat Genet Symp, Paestwn, Italy 1: 159-162.
Ogbonnaya FC, Subrahmanyam NC, Moullet 0, de Majnik J, Eagles HA, Brown JS, Eastwood RF, Kollmorgen J, Appels R and Lagudah ES (2001) Diagnostic DNA markers for cereal cyst nematode resistance in bread wheat. Aust J Agric Res 52: 1367-1374.
Payne PI (1987) Genetics of wheat storage proteins and the effect of allelic variation on bread-making quality. Ann Rev Plant Physiol 38:141-153.
Payne PI and Lawrance GJ (1983) Catalogue of alleles for the complex loci G1uA1, GluB1, GIuD1, which code for the high molecular weight subunits of glutenin in hexaploid wheat. Cereal Res Commun 11: 29-35.
Penner GA, Kerber ER and Larter EN (1986) The differentiation between chromosome 7D of Triticum aestivum cv Canthatch and Triticum tauschii as measured by chromosome pairing. Can J Genet Cytol 28: 385-389.
Pestsova EG, Borner A and Roder MS (2002) Development of a set of Triticum aestivum - Aegilops tauschii introgression lines. Hereditas 135: 139-143.
Pflueger LA, D'Ovidio R, Margiotta B, Pena R, Mujeeb Kazi A and Lafiandra D (2001) Characterization of high- and low-molecular weight glutenin subunits associated to the D genome of Aegilops tauschii in a collection of synthetic hexaploid wheats. Theor Appl Genet 103: 1293-1301.
Pritchard DJ, Hollington PA, Davies WP, Gorham JL, Diaz de Leon, and Mujeeb-Kazi A (2002) K+/ Na+ discrimination in synthetic hexaploid wheat lines: Transfer of the trait for K+/Na+ discrimination from Aegilops tauschii into a Triticum turgidium background. Cereal Res Commun 30: 261-267.
Rayburn AL and Gill BS (1987) Molecular analysis of the D genome of the Triticeae. Theor Appl Genet 73: 385-388.
Schachtman DP, Lagudah ES and Munns R (1992) The expression of salt tolera nce from Triticum tauschii in hexaploid wheat. Theor Appl Genet 84: 714-719.
Schachtman DP, Munns R and Whitecross MI (1991) Variation in sodium exclusion and salt tolerance in Triticum tauschii. Crop Sci 31: 992-997.
Seah S, Spielmeyer W, Jahier J, Sivasithamparam K and Lagudah ES (2000) Resistance gene analogs within an introgressed chromosomal segment derived from Triticum ventricosa that confers resistance to nematode and rust pathogens in wheat. Mol Plant-Microbe Interact 13: 334-341.
Spielmeyer W, Huang L, Bariana H, Laroche A, Gill BS and Lagudah ES (2000) NBS-LRR sequence is associated with leaf and stripe rust resistance on the end of homoeologous chromosome group 1S of wheat. Theor Appl Genet 101: 1139-1144.
Tsunewaki K (1966) Comparative gene analysis of common and its ancestral species. II. Waxiness, growth habit and awnedness. Japan Jour Bot 19: 175-229.
Tsunewaki K (1968) Origin and phylogenetic differentiation of common wheat revealed by comparative gene analysis. In: Finlay KW and Shepherd KW (eds.) Proc III Int Wheat Genet Symp, Canberra, pp. 71-85.
Tsunewaki K, Takumi S, Mon N, Achiwa T and Liu YG (1991) Origin of polyploid wheats revealed by RFLP analysis. In: Sasakuma T and Kinoshita T (eds.) Nuclear and Organellar Genomes of Wheat Species. Kihara Mem Found, Yokohama, pp. 31-39.
Turnbull KM, Turner M, Mukai Y, Yamainoto M, Morell MK, Appels R and Rahman S (2003) The organization of genes tightly linked to the Ha locus in Aegilops tauschii, the D-genome donor to wheat. Genome 46: 330-338.
von Bueren M (2001) Polymorphisms in two homoeologous gamma-gliadin genes and the evolution of cultivated wheat. Genet Resour Crop Evol 48: 205-220.
Yen C, Yang JL, Liu XD and Li LR (1983) The distribution of Aegilops tauchii Cosson in China with reference to the origin of the Chinese common wheat. In: Sakamoto S (ed.) Proc VI Int Wheat Genet Symp, Kyoto. Maruzen, Kyoto, pp. 55-58.
Zhang HB and Dvorak J (1992) The genome origin and evolution of hexaploid Triticum crassum and Triticum syriacum determined from variation in repeated nucleotide sequences. Genome 35: 806-814.
Zhang P, Dreisigacker S, Meichinger AE, Reif JC, Mujeeb Kazi A, Van Ginkel M, Hoisington D and Warburton ML (2005) Quantifying novel sequence variation and selective advantage in synthetic hexaploid wheats and their backcross-derived lines using SSR markers. Mol Breed 15: 1-10.
Zhao YH and Kimber G (1984) New hybrids with D-genome wheat relatives. Genetics 106: 509-515.
Zohary D and Feldman M (1962) Hybridization between amphiploids
and the evolution of polyploids in the wheat (Aegilops-Triticum) group.
Evolution 16: 44-61.