Memorial Issue, Wheat Information Service No.100
Genomics of tetraploid wheat domestication
S. Ayal1, R. Ophir2 and A. A. Levy1*
1Department of Plant Sciences, Weizmann Institute of Science, Rehovot,
76100, Israel
2Department of Biological Services, Bioinformatics Unit, Weizmann
Institute of Science, Rehovot, 76100, Israel
* Corresponding author: Avraham A. Levy
Plant Sciences, Weizmann Institute of Science, Rehovot 76100, Israel
E-mail address: avi.levy@weizmann.ac.il
Summary
The domestication of plants represents one of the most important events in the development of human culture. We have used cDNA microarrays to study the alterations in gene expression that occurred during wheat domestication and we have compared the range of variation in the transcriptome of wild ( Triticum turgidum sap. dicoccoides) versus domestic wheat (T. turgidum ssp. dicoccum and ssp. durum). The frequency of genes differentially expressed was 2.53% in young spikes. A high proportion of the genes that were up-regulated in domestic wheat were related to carbon metabolism. Genes preferentially expressed in the wild included several storage protein genes. Finally, we determined the variation in levels of gene expression for each of the 2,493 genes studied. About 23% of the genes were polymorphic in levels of expression in the different lines. The intra-specific variation in gene expression within ssp. dicoccoides was about four times higher than within the domesticated species. That reduction in variation during domestication is in the same range as that found at the genomic level, for nucleotide polymorphism. Nevertheless, we found that certain genes were more variables in the domestic varieties than in the wild. This suggests the possibility that selection under domestication led to the fixation of new mutations that did not previously exist in the wild. In summary, this work showed that high throughput expression analysis of genes provides much insight into evolutionary studies on the genetic structure of wild population, on biodiversity as well as on evolution under domestication.
Key words: wild emmer wheat, Triticum turgidum asp. dicoccoides, T. turgidum ssp. durum
Introduction
The domestication of plants represents one of the most important events in the development of human culture. This occurred about 12,000 years before present. Domestication of plants reflects the first step towards a whole new culture that developed around village, town and eventually city life.
Wheat was among the first and perhaps the most important plant species to be domesticated. Most scholars agree that wheat domestication, as well as that of barley, several legumes, flax and others occurred in the western arch of the Fertile Crescent, in what is known as the Levantine corridor (Israel, Jordan, Syria, Lebanon and southern Turkey) (Bar-Yosef 1998; Lev-Yadun et al. 2000; Otte et al. 1998; Salamini et al. 2002; Zohary and Hopf 2000) and is thus one of the truly great contribution of this area to humanity.
Wild emmer wheat, the "Mother of Wheat", Triticum turgidum ssp. dicoccoides, is a tetraploid species (2n=4x=28) containing the A and B genomes. This wild taxon is the progenitor of most domesticated wheats (Feldman 2001). As such, wild emmer is genetically very close to domesticated tetraploid (durum) wheat and to hexaploid (bread) wheat and the F1 hybrids between them are fully fertile and partially fertile, respectively.
The gene pool of wild emmer, being larger and richer than that of the cultivated wheat, contains many agronomically valuable alleles that are easily exploitable for wheat improvement (Feldman and Millet 1995). Already Aaronsohn, while discovering wild emmer in nature, noticed that certain specimens of this taxon contained several agronomically-important traits such as large grain size, ability to grow in relatively dry habitats, and resistance to rust. Aaronsohn believed that "the cultivation of wheat might be revolutionized by the utilization of wild emmer (Aaronsohn 1910).
In the last decades there has been a renewed interest in the germplasm of wild emmer as a source of agronomically-important traits. Detailed genetic studies of populations of wild emmer in situ indicate that this species is highly polymorphic (Anikster et al. 1991; Felsenburg et al. 1991; Huang et al. 1999; Levy et al. 1988; Nevo and Beiles 1989; Nevo et al. 1982) and has characteristics that would be valuable if transferred to domesticated wheat. Recent surveys of samples of wild emmer collected throughout its distribution area have confirmed Aaronsohn's findings that this wild wheat contains many agronomically-important alleles, such as genes for large grains and high grain protein percentage (Avivi 1979), desirable composition of storage proteins (Levy et al. 1988), disease resistance (Dinoor et al. 1991; Moseman et al. 1985), and increased grain yield and grain protein yield (Feldman and Millet 1995; Millet et al. 1998). While the utility of wild emmer is now recognized, the identification of useful wild genes and especially those that contribute to quantitative traits and the transfer into high-yielding cultivars remains a difficult task. Nevertheless, recent analysis of a broad range of traits, including QTLs was performed in wild emmer wheat, leading to the mapping of genes related to yield components as well as to genes related to domestication of emmer wheat (Peng et al. 2003).
While studying the process of plant domestication, one of the first questions that should be asked is, how different are wild progenitors from their domestic relatives? In wheat, the obvious examples of differences that can be noticed by analysis of visible phenotypes include spike fragility (shattering), an essential trait in the wild, versus non-fragile spikes in domesticated wheat, free-threshing grains (naked grains) in the domestic vs. hulled grains in wild wheat, erect plants and increased number of grains per spikelets, rapid and uniform germination, longer grain-filling period. All these traits, which are harmful in the wild, present obvious advantages to the early farmer, such as ease of harvest and higher yield It is likely that there are many additional traits, not visible to the eye, that are essential for survival in the wild, such as resistance to a broad range of abiotic and biotic stresses, loss of secondary metabolites that might have been used by the wild progenitor for preserving the grain in the soil or for symbiosis with microbes or efficiency in the uptake of nutrients that are less abundant in nature than in the farmer's field, etc. Some of these genes might become dispensable in the domestic types and thus might become mutated or silenced. Several studies have attempted to characterize and map the domestic genes in different plants; so far, only a few genes have been isolated. In wheat, the fragility genes were mapped to the 3AS and 3BS arms (Feldman, unpublished data), and to chromosomes 3A and 3B by Watanabe and Ikabata (2000), while Peng et al. (2003) mapped a gene that control brittle rachis (br) to chromosome 2A. In rice, four different QTLs were mapped for non-shattering of the seeds (Cai and Morishima 2002), and in barley the nonbrittle rachis genes 1 (btr1) and 2 (btr2) where mapped to chromosome 3H (Komatsuda et al. 2004). None of these genes has been identified in wheat.
Recently, DNA polymorphism surveys at the genome level are becoming common, with rapid advances in sequencing and genotyping technologies. One recent example is a work by the Doebley's group, which compared SSR markers in maize lines that were polymorphic versus those that were monomorphic among domestic lines (Vigouroux et al. 2002). The monomorphic genes were considered as candidates for selection during domestication.
The new tools of genomics (Schena et al. 1995), which allow the parallel and comparative analysis of the expression of many genes, have not yet been applied to study the process of domestication in wheat. Expression profiling using cDNA microarray is emerging as a powerful method to identify genes of interest in plants like the SAAT gene involved in strawberry flavor (Aharoni et al. 2000) or genes that are involved in response to different kinds of stresses (Reymond et al. 2000; Schenk et al. 2000). These tools have not yet been used to isolate important wheat genes. In grasses, the entire rice genome has been sequenced (Goff et al. 2002; Yu et al. 2002). Availability of the rice genome should facilitate the mapping of genes in cereals because gene sequence and order have been relatively conserved during grasses evolution (Gale and Devos 1998). In wheat, the large size of the genome (1.7 x 1010bp) is currently hindering full genome sequencing projects. Nevertheless in this crop, sequencing has focused on transcribed sequences and a total of > 600,000 expressed sequence tags (ESTs), (http:// wheat.pw.usda.gov/genome/), corresponding to the partial sequence of 128,088 unique different sequence (www.tigr.org/tigr-scripts/tgi/) are available. This sequencing effort concerns mostly cultivated bread wheat and there is no data on genes expressed in wild emmer wheat, the direct progenitor.
In this study, we have addressed the major alterations in the wheat transcriptome that are associated with the process of wheat domestication. We took a genomic approach to identify the genes whose expression was altered (up or down regulated) during domestication and to determine the range of variation in the wheat transcriptome of wild (Tnticum turgidum ssp. dicoccoides) versus domestic tetraploid wheat (Triticum turgidum ssp. dicoccum and ssp. durum). We discuss the utility of high throughput expression studies on the analysis of biodiversity as well as on evolution under domestication.
Materials and methods
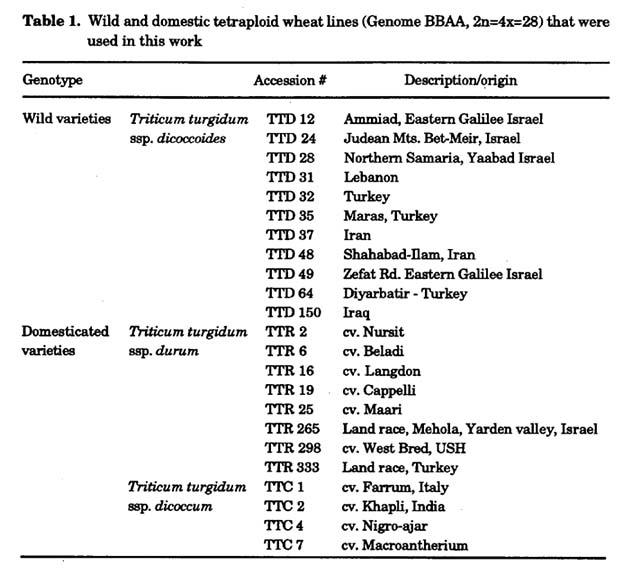
Plant materials used in the work: All the genetic materials used in this work were kindly provided by Prof. Moshe Feldman. A broad collection of wild and domestic lines from different parts of the Fertile Crescent was chosen in order to cover as much as possible variation. Eleven wild varieties (T. turgidum ssp. dicoccoides (TTD)) that cover a broad range of habitats and most of the variation in the species were chosen. Twelve domestic cultivars, 8 free-threshing (T. turgidum ssp. durum (TTR)) and 4 hulled (T. turgidum ssp. dicoccum (TTC)) from different parts of the world were chosen (Table 1). The dicoccum and the durum together, represent most of the variation within the domestic tetraploid species.
Growth conditions: All plants were grown in 3-liter pots in greenhouses during the summer period or in net-houses during winter.
AFLP and diversity analysis: The AFLP procedure was performed according to the protocol of Gibco BRL according to Vos et al. (1995), with minor modifications as previously described (Shaked et al. 2001).
In this work each AFLP band was considered to be a single locus. Autoradiographs were scored based on the presence or absence of bands at polymorphic loci generating matrix of 1's and 0's representing the presence or absence of a band respectively. Only those bands falling in the range of 50-500 bp were considered. These bands were used to generate a genetic similarity matrix with the JMP (JMP IN® Version 5) Statistical package, using the Hierarchical cluster with the ward method.
Amplified RNA (aRNA) : Total RNA was isolated from the relevant tissue for each experiment using the TRI (St. Paul) reagent method (Chomczynski 1993). Ten milligrams of total RNA were reverse transcribed using 1 ml (picomol) of T7 Oligo (dT) primer from Ambion (Cat#5710) in 20 ml reaction with 1 ml of M-MLV H- enzyme. Second strand cDNA was obtained using 15 ml of Poll buffer, 1 ml RnaseH, 2.5 ml DNTP's 25 mM in total volume of 150 ml, the reaction mixture was incubated for 2 h at 16°. After incubation, the cDNA was cleaned by phenol followed by chloroform and then phenol precipitation. The pellet was resuspended in 9 ml of DEPC treated water. In vitro transcription was done by adding the cDNA (8 ml), 6 ml of DNTP's 25 mM, 2 ml T7 RNA polymerase buffer, 0.5 ml dTT 400 mM, 1 ml Rnasin (biolab), 4 ml MgCl2 25 mM, and 2 ml T7 RNA polymerase (biolab). Each in vitro transcription was made from three independent reverse transcription reactions followed by three independent cDNA reactions. We took 2.7 ml from each cDNA reaction and pooled these samples in order to get the 8 ml needed for the in vitro transcription reaction.
Production of suppression subtraction hybridization libraries: Suppression subtraction hybridization (SSH) was developed for the generation of subtracted cDNA library. This method involves hybridization of the cDNA from one population (tester) to excess of cDNA from another population (driver) and then preferential amplification of the unhybridized fraction (Diatchenko et al. 1996). Two subtractive spikes cDNA libraries were obtained, using CLONTECH PCR-Select cDNA Subtraction Kit (CLONTECH Laboratories, Inc.). The first library was enriched with the genes from domesticated wheat spike T. turgidum ssp. durum (SSH1) and the second with genes from wild wheat spike T. turgidum ssp. dicoccoides (SSH2). Each RNA sample was made from a pool of 11 wild varieties or 8 domesticated varieties as described in Table 1. Tissues were collected from different stages of spike development, from young spikes, around one week after anthesis till mature spikes.
Microarrays production: A total of 2,493 cDNA clones were amplified by PCR using T3 and T7 primers. PCR (100 µl total volume) was prepared in a 96-well plate (Abgene, Surrey, UK), and amplified products were purified using EtOH precipitation (Hengen 1996). After purification, the samples were dried, resuspended in 15 µl of 50% DMS0 (200 to 400 ng DNA/µl), and transferred to a 384-format plate. Amplified cDNAs were applied to super amine slide using an arraying robot with 48(12 x 4) MicroQuill pins heads (BioRobotics, Ltd., Cambridge, UK) in 11x 10 subarrays. Each cDNA done was arrayed in two replicates at random positions. The slides were air-dried and then baked for 2 h at 80°C. cDNAs were UV cross-linked.
The cDNA clones that were spotted on the slides came from three sources: 192 ESTs were from the SSH library that was enriched for domesticated genes (SSH1), 2,108 ESTs were from the SSH library enriched for wild genes (SSH2) and 192 ESTs were derived from bread wheat seedlings that underwent a cold treatment. These bread wheat clones were kindly provided by the lab of Dr. Eliot Herman (USDA Beltsville).
Chip hybridization and analysis: The aRNA products were subjected to reverse transcription and then labeled with Cy3 and Cy5. For each biological repetition, at least two hybridization reactions with swapped dye labeling were performed.
Slides were incubated for 45 min at 42°C in preheated prehybridization buffer (5 x SSC, 0.1% SDS, and 1% BSA), washed in water and isopropanol, and dried by centrifugation. The probes (Cy3- and Cy5-labeled cDNA) were mixed, dissolved in 50 µl of ddw and mix with 50 µl of 2x hybridization buffer(50% formamide, 10% SSC, and 0.2% SDS) containing 15 µg of poly(A) DNA (Amersham Pharmacia Biotech) and 5 µg of yeast tRNA (Gibco BRL), denatured at 95°C for 3 min, and cooled on ice for 30 sec. The probe (100 µl) was applied directly to the slides (under a cover slip), which then were incubated in a hybridization chamber (TeleChem International, Sunnyvale, CA) at 42°C for 18 h. After hybridization, the slides were washed with 2 x SSC and 0.1% SDS for 5 min at 42°C, with 0.1 x SSC and 0.1% SDS for 10 min at room temperature, and then with 0.1 x SSC for 1 min, four times at room temperature, and finally dried quickly by centrifugation. Hybridized microarray slides were scanned immediately for fluorescence emission. Separate images for each fluorescence type (Cy3 or Cy5) were acquired using ScanArray 4000 software (Packard BioScience, Meridan, CT) at a resolution of 10 mm per pixel, adjusting the photomultiplier and laser power to achieve an optimal distribution of signals without minimal saturation.
Initial image analysis was performed using the QuantArray version 3 software using the histogram method. Data analysis was performed applying per-tip, per-spot and per chip normalization using Gene-Spring 6 software from Silicon Genetics. Significantly up-or down-regulated genes were filtered for expression ratios of 1.8 fold, and for t-test of P value <0.05. Further statistical analysis was done using the JMP program Version 5 Release 5.0.1 (JMP IN®). The overall population variation and the variation for each transcript within and between wild versus domesticated wheat were calculated, and the Log F ratio were determined.
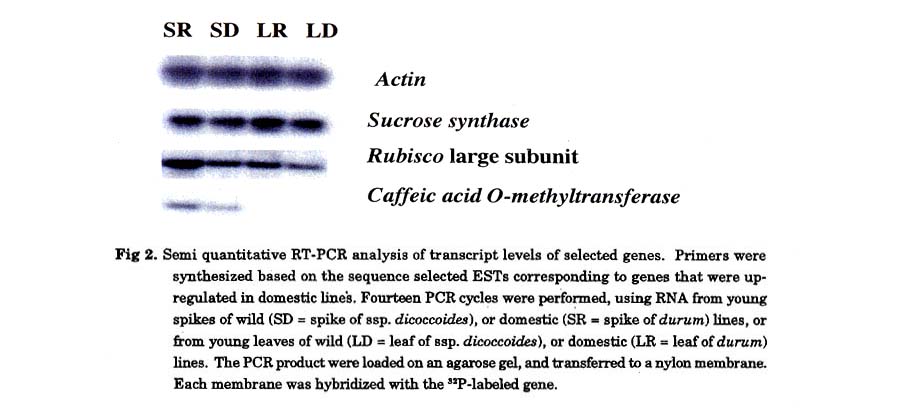
Semi-quantitative RT-PCR: Each EST that was chosen for verification was amplifled by 14 cycles of RT-PCR and run on a 1.5% agarose gel and transferred to a nylon membrane The cDNA was 32P-labeled as described (Feinberg and Vogelstein 1983), and was hybridize to a Hybond N+ membrane (Amersham, Buckinghamshire, UK) according to the manufacturer's recommendations. Reverse transcriptase (RT)-PCR was performed according to the SuperScript One-Step RT-PCR kit (GIBCO-BRL, Gaithersburg, MD) using the following PCR conditions: 5 min at 94°, 30 sec at 94°, 1 min at 52°, and 1 mm at 72° followed by 14 cycles (linear pass).
Results
Suppression subtraction hybridization libraries (SSH): We produced two suppression subtraction hybridization libraries (SSH) as described in the Materials and Methods. The first (SSH1) was enriched for domestic-specific genes (ssp. durum + ssp. dicoccum); while the second (SSH2) was enriched for wild-specific genes (ssp. dicoccoides). Around 2,000 clones were isolated from each library. In order to check the quality and redundancy of the library, 96 clones of SSH1 and 288 clones SSH2 were sequenced. 78% of the domesticated enriched library and 87.5% of the wild enriched library were single genes, while only 6% of SSH1 and 4% of SSH2 were contigs of 3 or more ESTs (Table 2).
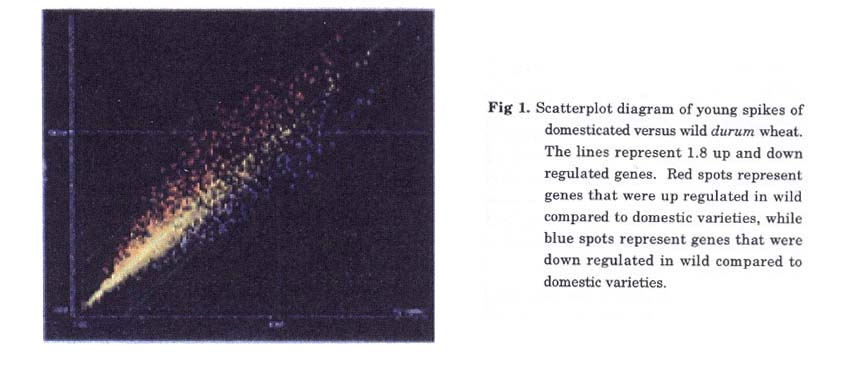
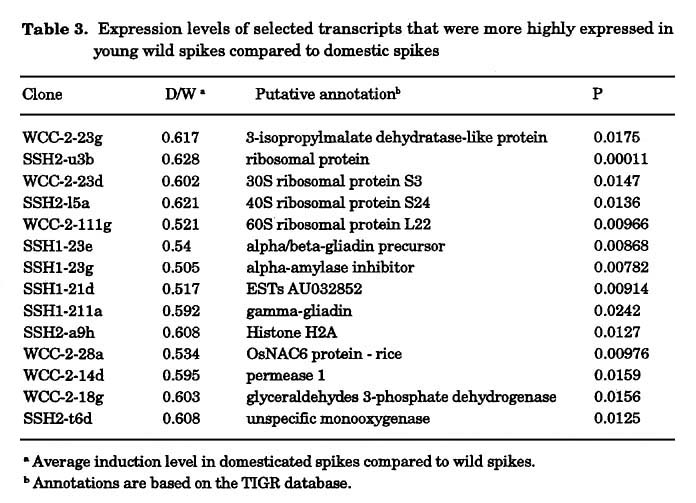
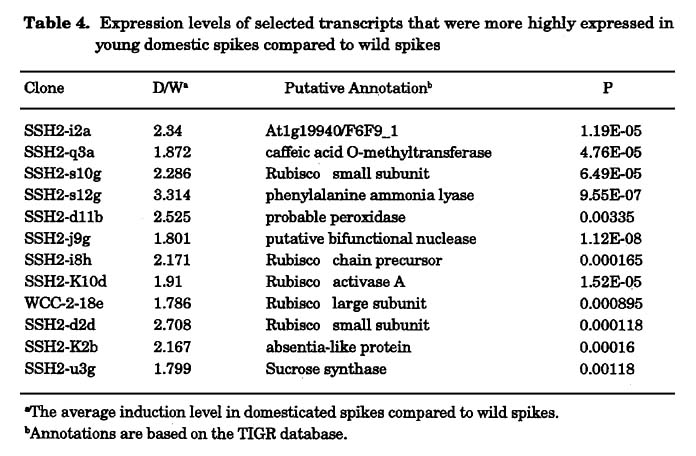
Expression profile of wild versus domesticated wheat in young spikes: RNA was extracted from a mix of different varieties of either wild or domesticated young spikes at the stage of one week after anthesis. A first pool was made out of 11 wild wheat accessions (Table 1a) and a second pool was prepared from 8 free-threshing domesticated (durum) lines (Table 1b). The pools of wild versus domesticated aRNA were labeled against each other in six replicas with dye swap. Genes that were up or down regulated by a factor of at least 1.8, in at least four of six replicas were identified (Fig. 1) and sent for sequencing and annotation. The expression levels of 63 genes, which represent 2.53% out of the 2,493 genes studied, were up or down regulated. Thirty-eight genes (1.52%) were up regulated (Table 3) and 24 genes (0.96%) were down regulated (Table 4) in the wild compared to the domesticated lines (Fig. 1). Most of the genes that were up regulated in the domesticated varieties are correlated with sugar metabolism, like the Rubisco large and small subunits and the sucrose synthase gene. We choose different genes for semi-quantitative RT-PCR in order to validate the microarray expression data (Fig 2).
Variation in gene expression (transcript level) versus genetic variation (DNA level) within and among turgidum wheat populations: In order to compare the variation in gene expression versus genomic variation within wild versus within domesticated gerniplasm, we estimated the level of genomic variation, namely DNA polymorphism, using AFLP. The variation in gene expression was determined using quantitative values for the expression of each gene in the different ecotypes obtained using micro arrays. These analyses were performed on plants grown in pots in a greenhouse under the same conditions.
For each sampling we used two pots, each pot containing two plants. Replicas were grown in random order. For measurements of the genomic variation, DNA was extracted from all plants. For gene expression measurements, tissues were collected from three-week-old seedlings and from young spikes, one week after anthesis and total RNA was extracted.
Analysis of DNA polymorphism (genomic variation): We examined 23 different varieties of tetraploid wheat (see Table 1), 11 wild varieties (T. turgidum ssp. dicoccoides (TTD)) from different parts of the fertile crescent and 12 domesticated lines, 8 free-threshing (T. turgidum ssp. durum (TTR)) and 4 non-free threshing (T. turgidum ssp. diccocum (TTC)). We measured genomic variation using AFLP with EcoRI and MseI restriction enzymes. Eight different primer combinations were used. A total of 700 bands were screened and out of these, 99 were polymorphic.
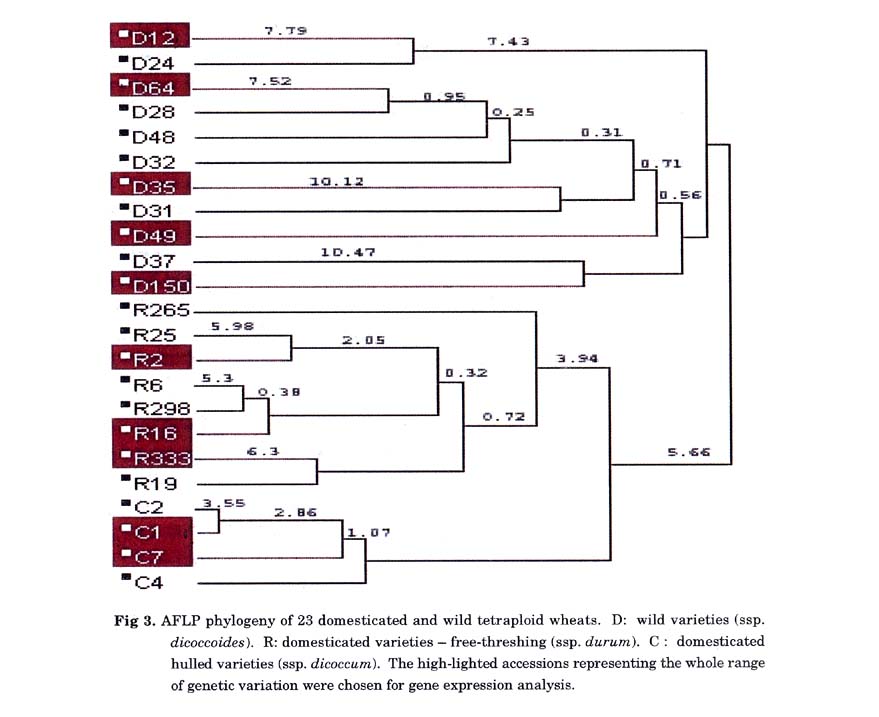
Among the wild population, 89 bands showed polymorphism whereas among the domesticated lines, only 57 bands showed polymorphism. Cluster analysis with the entire AFLP data divided all lines into two major branches (Fig 3), the wild ecotypes (ssp. dicoccoides) and the domesticated ecotypes. Within the domesticated lines there was one branch of the ssp. durum lines and another branch of the ssp. dicoccum lines. Ten lines, which represent different branches on the tree, were chosen for gene expression analysis (labeled in bold), 5 wild ecotypes, and 5 domestic lines, 3 of ssp. durum and 2 of ssp. dicoccum.
In addition, the genetic variance was calculated for each of the polymorphic loci from the AFLP analysis. This was done by giving a numerical value to each locus, namely 1 for the presence of the AFLP band and 0 for its absence. The frequency of monomorphic loci (variance = 0) was ∼4 fold higher in the domestic than in the wild wheat. The overall mean variance within the wild was 0.173 versus 0.107 among the domestic lines. This corresponds to a F value of F = 0.173/0.107 =1.62, p <0.01. In other words the genetic variance is 60% higher in the wild than in the domestic wheat.
Gene expression variation: We used a reference design experiment, namely, hybridization of each sample against a pool of the all other samples. Each sample was hybridized in two replicas with dye swap. Images and normalization were performed as described previously. The normalized data were further analyzed using the Jump statistics program. A one-way Nested ANOVA analysis was used in order to detect the variation within and between the wheat populations. The expression level of 577 genes (23%) were significantly different between lines within the same population at p value of 0.05. this number is 4.6 fold higher than the false positives expected under the null hypothesis when p = 0.05.
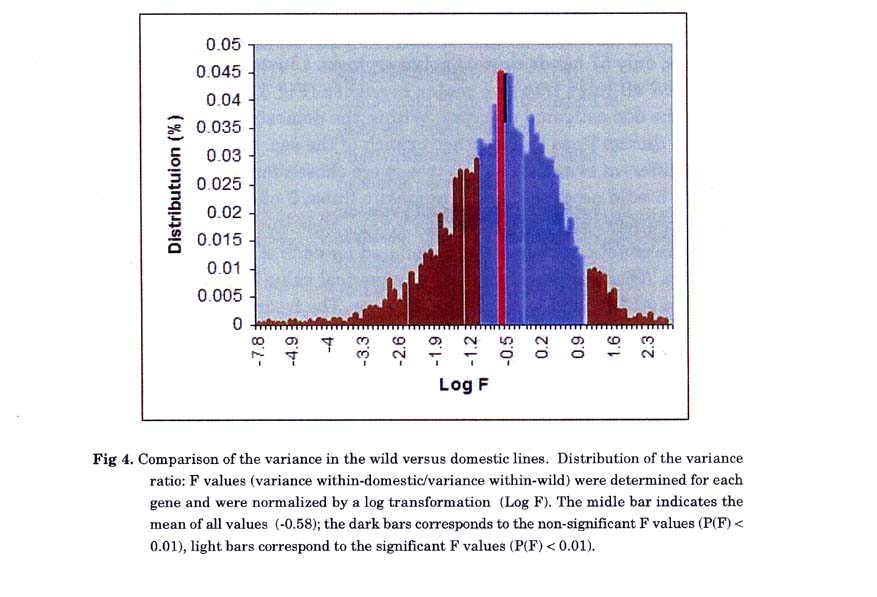
Comparison of variances in wild versus domestic wheat: We have compared the internal variation within-wild versus within-domestic wheat. A detailed analysis was done for the variation at the gene expression level, by calculating the variance at each locus within the wild and within the domestic group. We then determined the ratio (F values) between the two variances (Var (domestic)/Var (wild)). The ratio was normalized using a log transformation of the F values. Values of Log(F) = 0 correspond to identity in variation between wild and domestic; Log(F) >0 represent genes with a variance higher in the domestic varieties, while a negative value, Log(F) <0, represents genes with a higher variance in the wild varieties. The overall mean of log (F) is -0.58, indicating a higher ( ∼4 fold) variation in gene expression among the wild accessions (Fig. 4).
Discussion
Domestication of plants and animals has fascinated geneticists and evolutionary biologists starting from DeCandole, Mendel, Darwin and many other scientists. Early efforts were aimed at discovering the wild progenitors of domestic animals and plants. This was done using classical genetics and cytogenetics. Later on DNA markers were used to establish phylogenetic distances between wild and domestic types. Another recent field of investigation was the attempt to identify at the molecular level the key genes that were altered and selected by the process of domestication.
The novelty of our approach was to use a genome-wide analysis to determine in a non-biased manner the alterations in gene expression that occurred during domestication. Moreover, a bonus of our approach was to quantify for the first time in plants, the variation in gene expression among wild and domestic types. Earlier studies of plant populations had focused on polymorphism in nucleotide sequences, usually in non-coding DNA sequences. In this study, we have compared variation at the DNA and RNA levels in wild and domestic wheat; we asked what are the specific genes whose expression was altered (up or down regulated) during domestication? And what is the range of variation in the wheat transcriptome of wild (T. turgidum ssp. dicoccoides) versus domestic wheat (T. turgidum ssp. dicoccum and ssp. durum) ?
How different is the genetic makeup of wild versus domestic tetraploid wheat? A fundamental question that had not been addressed in previous studies is to what extent can we expect to find new genes in the wild that do not exist in the domestic? Comparing the sequences annotation of the genes to the existing ( www.tigr.org) wheat ESTs libraries, which are almost entirely derived from domestic wheat, we found that almost all sequenced clones (from both SSH1 and SSH2 libraries) had a "domestic" homolog EST in the wheat database. Only 5 "wild" EST's had no homology at all. Those genes might have a domestic homolog that did not show up yet among the existing ESTs. This could be the case for genes with very low transcription level like transcription factors. A second possibility is that those genes with no domestic homolog, correspond to wild-specific genes. We do expect that some genes from the wild might have been lost (or mutated) in domestic wheat, such as for example the fragility gene. However, these genes might be very few and would not be picked in our sequence analysis. One conclusion from this part is that the gene composition per se does not differ much between wild and domestic wheat. This is consistent with recent data of comparative genomics in plants and animals showing that related species contain essentially the same set of genes. This also supports the proposal of King and Wilson (1975) that evolution may essentially depend on variation in gene expression. Their proposal related to the evolution of species. Our sequencing data shows that it is certainly true for comparison between closely related species such as the ones analyzed here. In addition to this insight from sequence analysis, our work has addressed the rather unexplored differences in expression profiles between wild and domestic wheat.
Another control that is important to mention prior to the discussion of expression profiles is that the clones obtained through SSH did not show a bias in expression towards wild or domestic. This was checked by measuring the hybridization ratio between the two samples (wild and domestic). We did not find any bias toward wild or domesticated samples (data not shown). This satisfies the requirement for equal overall hybridization of the compared treatments as discussed by Quackenbush for proper microarray analysis (Quackenbush 2002). This, together with the fact that almost all our wild ESTs exist also in domesticated libraries, enable us to relate to our library as a normalized random collection of clones, rather than as a collection enriched for domestic or wild genes.
Expression profile of wild versus domesticated wheat in young spikes: The regions of DNA that affect. gene expression are highly variable. In the human genome, Stephens et al. (2001) found an average of one SNP approximately every 185 bases, meaning 0.6% polymorphic sites. Those naturally occurring polymorphic nucleotides can alter in vivo transcription rates as well as RNA stability. Thus, one might expect substantial variation in gene expression levels between individuals. As a result of their study on the fruit size gene evolution under domestication, Cong et al. (2002) proposed that much of the phenotypic diversity within a genus might be due to the changes within the non-coding sequences in the genome rather than structural point mutations in the genes coding region. Wang et al. (1999) found that the maize tb1, has only about 2% of the diversity found in teosinte for the 5' upstream region, but about 30% of the diversity found in teosinte for the protein-coding region. Those findings pinpoint that selection during domestication is associated with strongly reduced diversity in the non-transcribed region where regulatory sequences are typically found.
The natural variation in mRNA expression for large number of genes in wild versus domesticated population or within wild and within domesticated populations has not been measured in plants. Using the microarray technique, we are able to analyze a large number of genes in a single experiment. Note that finding polymorphism using this strategy is potentially more powerful than looking for the point mutations that may affect each gene. It is a high-throughput approach; the genes that are being compared are randomly chosen and present a random sample of the population.
We have used cDNA micro-arrays in order to obtain a genome-wide view of the alterations in gene expression that occurred during domestication. This microarray analysis measured the level of mRNA of more than 2,000 genes simultaneously without any particular assumption. In one set of experiments, RNA profiles in spikes from different stages of development from wild and domesticated T. turgidum wheat were compared. We found that the expression levels of 63 genes, which represent 2.53% of the total number of tested genes, were up or down regulated. Twenty-four genes (0.96%) were down regulated (Table 4) and 38 genes (1.52%) were up regulated (Table 3) in the wild compared to the domesticated lines (Fig. 1). Assuming that the tetraploid wheat genome contains approximately 40,000 genes, this means that approximately 1,000 genes are differentially expressed between, wild and domestic wheat and out of these, the expression of approximately 600 genes was reduced (i.e., lost, mutated, or silenced) by the domestication process. This figure is much higher than the Beadle's 5 gene difference between wild and domesticated corn (Beadle 1939, 1980). Obviously, it is probable that not all 600 less expressed genes are essential for survival in the wild. Conversely, it is also likely that it takes more than the few major genes that control visual phenotypic features (e.g., spike fragility) to make a species that can thrive in the wild. Many other genes (e.g., dormancy, nutrient uptake, hardiness, etc.) are probably involved.
Specific genes related to the domestication process: Among the genes that were up regulated in the domesticated varieties, we found that most of the genes correlate with carbon metabolism, like the Rubisco large and small subunit and the sucrose synthase (SuSy). Yield increase in domestic wheat has been achieved mostly through the increase of the carbohydrate fraction (resulting in an associated decrease in protein percent in the grain) (Avivi 1979; Wasserman et al. 1995). Alterations in the activity of those genes, expressed both in wild and domestic varieties, has probably occurred as the result of a long selection process. These quantitative changes are not usually considered as key events in domestication and were missed by previous studies.
The enhanced Rubisco RNA levels in domestic wheat were associated with an increase in protein expression as tested by SDS-PAGE in both the flag leaf and in immature spikes. Remarkably, this supports earlier findings by Galili et al. (1998) showing that specific wheat Rubisco subunits had a higher expression in domestic compared to wild wheat. They also showed that in domestic wheat, there was a reduction of genetic variation at this locus suggesting that it was an important target for yield selection during domestication. Considering that Rubisco is the most abundant protein in the green tissues, this increase is very impressive. C3 photosynthesis efficiency, measured at higher illumination, is positively correlated with Rubisco content (Stitt et al. 1991). Additional works also point to the activity of Rubisco being a limiting factor of photosynthesis (Farquhar et al. 1980). Thus, decreasing Rubisco content decreased photosynthesis nearly proportionately at ambient C02 and high light (Hudson et al. 1992; Lauerer et al. 1993). Another gene that was up regulated in the domestic varieties and might correlate with carbon metabolites is the surcose synthase gene SuSy. Two key enzymes involved in sucrose to starch conversion are known to be subject to transcriptional regulation, these are SuSy and AGPase. SuSy is the first step in the pathway from sucrose to starch, catalysing the conversion of sucrose and UDP to UDPGlc and fructose via a readily reversible reaction (Geigenberger and Stitt 1993), its expression being increased by sucrose, anaerobiosis and wounding (Salanoubat and Belliard 1989; Zeng et al. 1998). Altogether, those results suggest that the enhanced expression of both the Rubisco and the SuSy during domestication contributed to the breeding of a more efficient "source" and might have contributed to the increase in starch that forms the bulk of the dry weight in the grain's endosperm, thus for the increase in total grain yield. Furthermore, increasing the Rubisco also in the spike may reflect a longer period of grain filling. In maize, Whitt et al. (2002) evaluated the impact of selection across the starch metabolism by comparing the DNA sequence of six genes involved in the starch pathway. They found that in three of the genes that were checked, brittle2 (bt2), sugary1(su1), and amylose extender1(ae1), the level of diversity in the domesticate maize was low compared to the wild relative, suggesting selection for these loci during domestication and thus supporting the wheat data.
Another EST that was highly expressed in the domestic varieties is the caffeic acid 0 methyltransferase. The caffeic acid O-methyltransferase is involved in different methylation pathways in lignin biosynthesis (Jouanin et al. 2000; Ye and Varner 1995), its higher activity in spikes of domestic wheat may contribute to the non-fragility and the strength of the spike.
On the other hand several genes were up regulated in the wild compared to the domestic wheat. This includes members from the gliadin family. This may result from two factors: the high protein content found in wild tetraploid wheat (up to 30%) compared to domesticated wheat (up to 14%) (Avivi 1979) and the higher variability in the types of storage proteins in the wild compared to the domesticated wheat where several storage proteins were lost (diploidization) during the process of domestication (Levy et al. 1988). Another set of genes that were up-regulated in the wild compared to the domestic varieties related to various abiotic and biotic stresses, e.g. the unspecific mono-oxygenase, which is related to the cytochrome P450 gene superfamily. This gene family is related to the response to oxidation damage (Narusaka et al. 2004). Another stress-related gene is Permease 1. This gene is related to catalysis of the stereospecific transfer of a substrate across a biological membrane. Members from this family have been shown to be involved in nutrition uptake (Desimone et al. 2002), and in salt stress response (Rentsch et al. 1996). Stress related genes are interesting for improving the hardiness of cultivated plants via transfer of relevant genes from the wild into the cultivated germplasm.
Variation in gene expression versus genomic variation within and among durum wheat populations: During the processes of domestication, usually only a small part of the wild gene pool is considered to have contributed to the diversity among domestic varieties. The variability within the domesticated varieties is considered as narrow, while the variation within the wild is considered much broader (Tanksley and McCouch 1997). In wheat, in the last decades there has been a renewed interest in the germplasm of wild emmer as a source of agronomically-important traits (Feldman and Sears 1981). Detailed genetic studies of populations of wild emmer indicate that this species is highly polymorphic (Anikster et al. 1991; Felsenburg et al. 1991; Huang et al. 1999; Levy et al. 1988; Nevo and Beiles 1989; Nevo et al. 1982). These studies have also addressed the phylogenetic relationship between wild and, domestic wheat using a variety of molecular methods: chloroplast DNA (Matsuoka et al. 2002; Provan et al. 2004), AFLPs (Ozkan et al. 2002) and microsatellite (Maccaferri et al. 2003).
In this work, we have studies the variability among T. turgidum species using both DNA polymorphism data, via AFLP, and using gene expression data via cDNA microarrays. This analysis enables for the first time to compare the two types of variabilities. While genetic variability measures any SNP or Indel variation within the genome, the microarray analysis we performed analyzed quantitative differences in gene expression that might result from variation in the regulatory regions of the gene or in patterns of expression of transcription factors.
We proposed to answer two questions. First, what proportions of genes are differentially expressed between individuals within the same germplasm (wild ecotypes or domestic varieties)? Second, how many genes are differentially expressed between the two types of germplasm (wild versus domestic)? To address these questions, we applied ANOVA methods to the log normalized data. Using the ANOVA we didn't depend on assessing ratios of fluorescent signals, whereby only large differences can be detected. Instead, like previous study (Jin et al. 2001; Oleksiak et al. 2002) we investigated which genes showed statistically significant variations in expression. This analysis was done using robust statistical tests such as the F test that compares ratio between variances (Sokal and Rohlf 1981).
We have studied the alterations in gene expression, in the early stages of spike development that occurred during wheat domestication. We have calculated the variation in gene expression between wild ecotypes or between domestic varieties, for each of the 2,493 genes studied. The expression level of 577 genes (23%) was significantly variable between wild ecotypes or between domestic varieties at P value <0.05. This number is 4.6 fold higher than the false positives expected under the null hypothesis when P = 0.05. The proportion of loci significantly variable in expression between wild ecotypes or between domestic varieties is similar to the percentage of loci that differ significantly in expression between different strains of yeast (24%) (Brem et al. 2002), the percentage of loci that show non-zero variance in Drosophila melanogaster (25%) (Jin et al. 2001) and the percentage of loci differ significantly in expression between natural populations of teleost fish (18%) (Oleksiak et al. 2002). On the average, the variation within ssp. dicoccoides was higher than within the domestic germplasm at both the DNA level (60%) and at the gene expression level (∼4 fold). It is interesting to note that overall, although the wild varieties contain a larger variation than the domestic varieties, we found a new variation that exist in the domestic varieties, and is absent in the wild. Among the group of genes that contain a new variation in the domestic varieties that does not exist in the wild varieties, we find members from the glutenin family, the sucrose-6F-phosphate, and some proteins with unknown function. ESTs that relate to sucrose metabolism are noticeable. This points on high selection forces related to quality and yield, which might have led to the fixation of new mutations in domestic types and thus to a new variation that did not previously exist in the wild.
Concluding remarks
We have studied the alterations in gene expression that occurred during wheat domestication, taking a genomic approach to address two issues: (i) What is the extent of varation in gene expression that can be attributed to domestication, (ii) what are the specific genes whose expression was altered (up or down regulated) during domestication? (iii) what is the range of variation in the wheat transcriptome of wild (T. turgidum ssp. dicoccoides) versus domestic wheat (T. turgidum ssp. dicoccum and ssp. durum) and (iv) what is the correlation between DNA and RNA variation. Earlier studies have focused on specific genes that were known in advance to have been affected by the domestication process while we took an unbiased approach to compare wild versus domesticated wheat. These studies include for the first time cDNA microarrays, in order to obtain a genome wide view of the alterations in gene expression that occurred during domestication.
The frequency of genes differentially expressed between wild and domesticated wheat was 2.53% in young spikes. A high proportion of the genes that were found to be up regulated in domestic wheat were related to sugar metabolism. Genes that were preferentially expressed in the wild versus domestic wheat were isolated and included several storage protein genes. Finally we determined the variation in levels of gene expression for each of the 2,493 genes, among wheat lines. About 23% of the genes were polymorphic in expression in the different lines. The intra-specific variation in gene expression within asp. dicoccoides was about four times higher than within the domesticated cultivars. Interestingly, this reduction in variation during domestication is in the same range as that found at the genomic level, for nucleotide polymorphism.
One of the difficulties to analyze variation in gene expression in different genotypes is that the environmental conditions have to be kept uniform to ensure that the different genotypes do not grow in a different environment. Future experiments should be carried in uniform conditions e.g. phytotron or growth chambers. This is of course one of the limitations of studies on gene expression, namely that keeping a fixed environment is the only way to expose differences in expression that have a genetic rather than environmental basis, on the other hand, we miss specific interaction with the natural environment.
Future works should address the type of molecular changes (cis versus trans) related to changes in gene expression. This can be achieved by selecting the genes that were differentially expressed and sequencing of their upstream regulatory regions. Another approach will be to look for alterations in the expression of transcription factors that regulate these genes. In summary, we propose that microarray expression analysis should become a mainstream tool to analyze wild germplasm, to study the evolution of plants and to better analyze the impact of selection for specific mutations (e.g. the fragility genes) verus more quantitative changes that might have targeted regulatory components.
Acknowledgements
We thank Prof. Moshe Feldman for providing germplasm as well as for fruitful and stimulating discussions and for editing of the manuscript. This work was supported by a grant from the Israeli Science Foundation, BIKURA program. A.A.L holds the Gilbert de Botton chair of Plant Sciences.
References
Aaronsohn A (1910) Agricultural and botanical explorations in Palestine. Bull Plant Industry 180:1 63.
Aharoni A, Keizer LC, Bouwmeester HJ, Sun Z, Alvarez-Huerta M, Verhoeven HA, Blaas J, van Houwelingen AM, De Vos RC, van der Voet H, Jansen RC, Guis M, Mol J, Davis RW, Schena M, van Tunen AJ and O'Connell AP (2000) Identification of the SAAT gene involved in strawberry flavor biogenesis by use of DNA microarrays. Plant Cell 12: 647-662.
Anikster Y, Eshel A, Ezrati S and Horovitz A (1991) Patterns of phenotypic variation in wild tetraploid wheat at Ammiad Israel J Bot 40: 397-418.
Avivi L (1979) High grain protein content in wild tetraploid wheat Triticum dicoccoides Korn. Proc V Int Wheat Genet Symp, New Delhi 1: 372-380.
Bar-Yosef 0(1998) On the nature of transitions: The Middle to Upper Palaeolithic and the Neolithic revolution. Camb Archaeol J 8: 141-163.
Beadle GW (1939) Teosinte and the origin of maize. J Hered 30: 245-247.
Beadle GW (1980) The ancestry of corn. Sci Am 242: 112-119.
Brem RB, Yvert G, Clinton R and Kruglyak L (2002) Genetic dissection of transcriptional regulation in budding yeast. Science 296: 752-755.
Cai W and Morishima H (2002) QTL clusters reflect character associations in wild and cultivated rice. Theor Appl Genet 104: 1217-1228.
Chomczynski P (1993) A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. Biotechniques 15: 532-534, 536-537.
Cong B, Liu J and Tanksley SD (2002) Natural alleles at a tomato fruit size quantitative trait locus differ by heterochronic regulatory mutations. Proc Natl Acad Sci USA 99: 13606-13611.
Desimone M, Catoni E, Ludewig U, Hilpert M, Schneider A, Kunze R, Tegeder M, Frommer MM and Schumacher K (2002) A novel superfamily of transporters for allantoin and other oxo derivatives of nitrogen heterocycic compounds in Arabidopsis. Plant Cell 14: 847-856.
Diatchenko L, Lau YF, Campbell AP, Chenchik A, Moqadam F, Huang B, Lukyanov K, Gurskaya N, Sverdlov ED and Siebert PD (1996) Suppression subtractive hybridization: a method for generating differentially regulated or tissue-specific cDNA probes and libraries. Proc Natl Acad Sci USA 93: 6025-6030.
Dinoor A, Eshed N, Ecker R, Gerechter-Amitai ZK, Solel Z, Manisterski J and Anikster Y (1991) Fungal diseases of wild tetraploid wheat in a natural stand in northern Israel. Israel J Bot 40: 481-500.
Farquhar GD, von Caemmerer S and Berry JA (1980) A biochemical model of photosynthetic C02 assimilation in leaves of C-3 species. Planta 149: 78-90.
Feinberg AP and Vogelstein B (1983) A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem 132:6-13.
Feldman M (2001) The origin of cultivated wheat. In: Bonjean A and Angus W (eds) The World Wheat Book. Lavoisier Tech & Doc, Paris.
Feldman M and Millet E (1995) Methodologies of identification, allocation and transfer of quantitative genes from wild emmer into cultivated wheat. Proc VIII Int Wheat Genet Symp, Beijing 1: 19 27.
Feldman M and Sears ER (1981) The wild gene ressources of wheat. Sci Am 244:102-112.
Felsenburg T, Levy AA, Galili G and Feldman M (1991) Polymorphism of high-molecular-weight glutenins in wild tetraploid wheat - Spatial and temporal variation in a native site. Israel J Bot 40: 451479.
Gale MD and Devos KM (1998) Comparative genetics in the grasses. Proc Natl Acad Sci USA 95: 1971-1974.
Galili S, Avivi Y and Feldman M (1998) Differential expression of three RbcS subfamilies in wheat. Plant Sci 139:185-193.
Geigenberger P and Stitt M (1993) Sucrose synthase catalyses a readily reversible reaction in developing potato tubers and other plant tissues. Planta 189: 329-339.
Goff SA, Ricke D, Lan TH, Presting G, Wang R, Dunn M, Glazebrook J, Sessions A, Oeller P, Varma H, Hadley D, Hutchinson D, Martin C, Katagiri F, Lange BM, Moughamer T, Xia Y, Budworth P, Zhong J, Miguel T, Paszkowski U, Zhang S, Colbert M, Sun WL, Chen L, Cooper B, Park S, Wood TC, Mao L, Quail P, Wing R, Dean R, Yu Y, Zharkikh A, Shen R, Sahasrabudhe S, Thomas A, Cannings R, Gutin A, Pruss D, Reid J, Tavtigian S, Mitchell J, Eldredge G, Scholl T, Miller RM, Bhatnagar S, Adey N, Rubano T, Thsneem N, Robinson R, Feldhaus J, Macalma T, Oliphant A and Briggs S(2002) A draft sequence of the rice genome (Oryza sativa L ssp.japonica). Science 296:92-100.
Hengen PN (1996) Carriers for precipitating nucleic acids. Trends Biochem Sci 21:224-225.
Huang L, Millet E, Rong JK, Wendel JF, Anikster Y and Feldman M (1999) Restriction fragment length polymorphism in wild and cultivated tetraploid wheat. Israel J Plant Sci 47:213-224.
Hudson GS, Evens JR, von Caemmerer S, Arvidsson YBC and Andrews TJ (1992) Reduction of ribulose-1,5-bisphosphate carboxylase oxygenase content by antisense RNA reduces photosynthesis in transgenic tobacco plants. Plant Physiol 98: 294-302.
Jin W, Riley RM, Wolfinger RD, White KP, Passador-Gurgel G and Gibson G (2001) The contributions of sex, genotype and age to transcriptional variance in Drosophila melanogaster. Nat Genet 29: 389-395.
Jouanin L, Goujon T, de Nadai V, Martin MT. Mila I, Vallet C, Pollet B, Yoshinaga A, Chabbert B, Petit-Conil M and Lapierre C (2000) Lignification in transgenic poplars with extremely reduced caffeic acid 0-methyltransferase activity. Plant Physiol 123: 1363-1374.
King MC and Wilson AC (1975) Evolution at two levels in humans and chimpanzees. Science 188: 107-116.
Komatsuda T, Maxim P, Senthil N and Mano Y (2004) High-density AFLP map of nonbrittle rachis 1 (bb-1) and 2 (btr2) genes in barley (Hordeum vulgare L.). Theor Appl Genet 109: 986-995.
Lauerer M, Saftic D, Quick WP, Labate C, Fichtner K, Schulze ED, Rodermel SR, Bogorad L and Stitt M (1993) Decreased ribulose-1,5-bisphosphate carboxylase-oxygenase in transgenic tobacco transformed with antisense rbcs. VI. Effect on photosynthesis in plants grown at different irradiance. Planta 190: 332-345.
Levy AA, Galili G and Feldman M (1988) Polymorphism and genetic control of high molecular weight glutenin subunits in wild tetraploid wheat Triticum turgidum var. dicoccoides. Heredity 61:63 72.
Lev-Yadun S, Gopher A and Abbo S (2000) Archaeology. The cradle of agriculture. Science 288: 1602 1603.
Maccaferri M, Sanguineti MC, Donini P and Tuberosa R (2003) Microsatellite analysis reveals a progressive widening of the genetic basis in the elite durum wheat germplasm. Theor Appl Genet 107:783-797.
Matsuoka Y, Yamazaki Y, Ogihara Y and Tsunewaki K (2002) Whole chloroplast genome comparison of rice, maize, and wheat: implications for chloroplast gene diversification and phylogeny of cereals. Mol Biol Evol 19: 2084-2091.
Millet E, Rong JK and Feldman M (1998) Production of wild emmer recombinant substitution lines in a modern bread wheat cultivar and their use in wheat mapping. Proc IX Int Wheat Genet Symp, Saskatoon 1: 127-130.
Moseman JG, Nevo E, Gerechter-Amitai ZK, El-Morshidy MA and Zohary D (1985) Resistance of Triticum dicoccoides collected in Israel to infection with Puccinia recondita tritici. Crop Sci 25: 262-265.
Narusaka Y, Narusaka M, Seki M, Umezawa T, Ishida J, Nakajima M, Enju A and Shinozaki K (2004) Crosstalk in the responses to abiotic and biotic stresses in Arabidopsis: Analysis of gene expression in cytochrome P450 gene superfamily by cDNA microarray. Plant Mol Biol 55: 327 342.
Nevo E and Beiles A (1989) Genetic diversity of wild emmer wheat in Israel and Turkey - Structure, evolution, and application in breeding. Theor Appl Genet 77: 421-455.
Nevo E, Golenberd EM, Beiles E, Brown AHD and Zohary D (1982) Genetic diversity and environmental association of wild wheat T. dicoccoides in Israel. Theor Appl Genet 62: 241-254.
Oleksiak MF, Churchill GA and Crawford DL (2002) Variation in gene expression within and among natural populations. Nat Genet 32: 261-266.
Otte M, Yalcinkaya I, Kozlowski J, Bar-Yosef 0, Lopez Bayou I and Taskiran H (1998) Long-term technical evolution and human remains in the Anatolian palaeolithic. J Hum Evol 34:413-431.
Ozkan H, Brandolini A, Schafer-Pregl R and Salamini F (2002) AFLP analysis of a collection of tetraploid wheats indicates the origin of emmer and hard wheat domestication in southeast Turkey. Mol Biol Evol 19: 1797.1801.
Peng J, Ronin Y, Fahima T, Roder MS, Li Y, Nevo E and Korol A (2003) Domestication quantitative trait loci in Triticum dicoccoides, the progenitor of wheat. Proc Natl Acad Sci USA 100: 2489 2494.
Provan J, Wolters P, Caidwell KH and Powell W (2004) High-resolution organellar genome analysis of Triticum and Aegilops sheds new light on cytoplasm evolution in wheat. Theor Appl Genet 108:1182-1190.
Quackenbush J (2002) Microarray data normalization and transformation. Nat Genet 32 Suppl: 496- 501.
Rentsch D, Hirner B, Schmelzer E and Frommer WB (1996) Salt stress-induced proline transporters and salt stress-repressed broad specificity amino acid permeases identified by suppression of a yeast amino acid permease-targeting mutant. Plant Cell 8: 1437-1446.
Reymond P, Weber H, Damond M and Farmer EE (2000) Differential gene expression in response to mechanical wounding and insect feeding in Arabidopsis. Plant Cell 12: 707-720.
Salamini F, Ozkan H, Brandolini A, Schafer-Pregl R and Martin W (2002) Genetics and geography of wild cereal domestication in the Near East. Nat Rev Genet 3: 429-441.
Salanoubat M and Belliard G (1989) The steady-state level of potato sucrose synthase mRNA is dependent on wounding, anaerobiosis and-sucrose concentration. Gene 84: 181-185.
Schena M, Shalon D, Davis RW and Brown P0 (1995) Quantitative monitoring of gene expression patterns with a complementary DNA microarray. Science 270: 467-470.
Schenk PM, Kazan K, Wilson I, Anderson JP, Richmond T, Somerville SC and Manners JM (2000) Coordinated plant defense responses in Arabidopsis revealed by microarray analysis. Proc Natl Acad Sci USA 97: 11655-11660.
Shaked H, Kashkush K, Ozkan H, Feldman M and Levy AA (2001) Sequence elimination and cytosine methylation are rapid and reproducible responses of the genome to wide hybridization and allopolyploidy in wheat. Plant Cell 13: 1749-1759.
Sokal RR and RohIf FJ (1981) Biometry. W.H. Freeman, New York.
Stephens JC, Schneider JA, Tanguay DA, Choi J, Acharya T, Stanley SE, Jiang R, Messer CJ, Chew A, Han JH, Duan J, Carr JL, Lee MS, Koshy B, Kumar AM, Zhang G, Newell WR, Windemuth A, Xu C, Kalbfleisch TS, Shaner SL, Arnold K, Schulz V, Drysdale CM, Nandabalan K, Judson RS, Ruano G and Vovis GF (2001) Haplotype variation and linkage disequilibrium in 313 human genes. Science 293: 489-493.
Stitt M, Quick WP, Schurr U, Schuize ED, Rodermel SR and Bogorad L(1991) Decreased ribulose-1,5-bisphosphate carboxylase-oxygenase in transgenic tobacco transformed with antisense rbcs. 2. flux control coefficients for photosynthesis in varying light, C02 and air humidity. Planta 183: 555-566.
Tanksley SD and McCouch SR (1997) Seed banks and molecular maps: unlocking genetic potential from the wild. Science 277: 1063-1066.
Vigouroux Y, McMullen M, Hittinger CT, Houchins K, Schulz L, Kresovich S, Matsuoka Y and Doebley J (2002) Identifying genes of agronomic importance in maize by screening microsatellites for evidence of selection during domestication. Proc Natl Acad Sci USA 99:9650-9655.
Vos P, Hogers R, Bleeker M, Reijans M, Van delee T, Hornes M, Frijters A, Pot J, Peleman J, Kuiper M, Zabeau M (1995) AFLP - a new technique for DNA fingerprinting. Nucleic Acids Res 23: 4407-4414.
Wang RL, Stec A, Hey J, Lukens L and Doebley J (1999) The limits of selection during maize domestication. Nature 398: 236-239.
Wasserman BP, Harn C, Mu-Forster C and Huang R (1995) Progress toward genetically modified starches. Cereals Food World 40: 810-817.
Watanabe N and Ikabata N (2000) The effects of homoeologous group 3 chromosomes on grain colour dependent seed dormancy and brittle rachis in tetraploid wheat. Euphytica 115: 215-220.
Whitt SR, Wilson LM, Tenaillon MI, Gaut BS and Buckler IV ES (2002) Genetic diversity and selection in the maize starch pathway. Proc Natl Acad Sci USA 99: 12959-12962.
Ye ZH and Varner JE (1995) Differential expression of two 0-methyltransferases in lignin biosynthesis in Zinnia elegans. Plant Physiol 108: 459-467.
Yu J, Hu S, Wang J, Wong GK, Li S, Liu B, Deng Y, Dai L, Zhou Y, Zhang X, Cao M, Liu J, Sun J, Tang J, Chen Y, Huang X, Lin W, Ye C, Tong W, Cong L, Geng J, Han Y, Li L, Li W, Hu G, Li J, Liu Z, Qi Q, Li T, Wang X, Lu H, Wu T, Zhu M, Ni P, Han H, Dong W, Ren X, Feng X, Cui P, Li X, Wang H, Xu X, Zhai W, Xu Z, Zhang J, He S, Xu J, Zhzang K, Zheng X, Dong J, Zeng W, Tao L, Ye J, Tan J, Chen X, He J, Liu D, Tian W, Tian C, Xia H, Bao Q, Li G, Gao H, Cao T, Zhao W, Li P, Chen W, Zhang Y, Hu J, Liu S, Yang J, Zhang G, Xiong Y, Li Z, Mao L, Zhou C, Zhu Z, Chen R, Hao B, Zheng W, Chen S, Guo W, Tao M, Zhu L, Yuan L and Yang H (2002) A draft sequence of the rice genome (Oryza sativa L. ssp. indica). Science 296: 79-92.
Zeng Y, Wu Y, Avigne WT and Koch KE (1998) Differential regulation of sugar-sensitive sucrose synthases by hypoxia and anoxia indicate complementary transcriptional and posttranscriptional responses. Plant Physiol 116: 1573-1583.
Zohary D and Hopf M (2000) Domestication of Plants in the Old
World. Oxford Univ. Press, Oxford.