Memorial Issue, Wheat Information Service No.100
Perspectives in molecular cytogenetics of wheat
Yasuhiko Mukai
Laboratory of Plant Molecular Genetics, Division of Natural
Science, Osaka Kyoiku University, Kashiwara, Osaka 582-8582, Japan
E-mail: ymukai@cc.osaka-kyoiku.ac.jp
Key words: molecular cytogenetics, fluorescence in situ hybridization (FISH), genomic in situ hybridization (GISH), chromosome, extended DNA fiber, physical mapping, gene visualization, agronomically important gene
Introduction
Autoradiographic detection of isotope-labeled specific DNA sequences on wheat chromosomes by in situ hybridization (ISH) (Appels et al. 1980; Bedbrook et al. 1980; Gerlach and Peacock 1980) made a beginning of the new era of molecular cytogenetics in wheat research a quarter century ago. Rayburn and Gill (1985) first applied non-isotopic ISH techniques on plant chromosomes using biotin-labeled probes for mapping of repetitive DNA sequences. ISH was further improved with the advent of non-isotopic fluorescent reporter molecules for labeling of DNA in 1980s leading to the development of fluorescence in situ hybridization (FISH) techniques (Langer-Safer et al. 1982). FISH methods have advantage over hybridization with isotope-based probes, including longer probe stability, speed, higher sensitivity; spatial resolution and simultaneous detection of more than one probe (Mukai 1996, 2004). Yamamoto and Mukai (1989) were the first to apply the FISH technique in wheat that was published in Wheat Information Service in the 69th number. It is now possible to physically visualize genes and DNA sequences under microscope on extended DNA fibers prepared from interphase / dividing nuclei. The genomic in situ hybridization (GISH) has provided new dimensions to accurately underpin the genome donors in natural polyploids based on direct genomic painting, omitting the need to test the validity by raising artificial hybrids. FISH techniques have since revolutionized molecular cytogenetics in general, and wheat molecular cytogenetics in particular, opening newer opportunities in plant breeding. The present article invigorates the progress made in wheat molecular cytogenetics tracing the developments in a prospective multidisciplinary perspective for the 21st century.
Physical mapping of repeated sequences
Repeated sequences can be divided into two types based on their organization and distribution pattern. One type constitutes tandem arrays localized as clusters along the chromosomes, and the other type comprises of dispersed repeats that are interspersed with unrelated repeats and are distributed over the entire genome. In the tribe Triticeae, repeated sequences such as pSc119.2, pSc74, pAs1, pHvG38, and pHvA14 could be used as chromosome markers / cytological landmarks having value in chromosome identification (Mukai et al. 1992; Rayburn and Gill 1986; Tsujimoto et al. 1997). Multicolor FISH using pSc119.2 and pAs1 enabled identification of all B- and D-genome chromosomes, and chromosomes 1A, 4A, and 5A of hexaploid wheat (Mukai et al. 1993a). Using pAs1 and pHvG38, all 21 chromosomes of hexaploid wheat can be identified (Pedersen and Langridge 1997). Dispersed sequences such as pSc119.1, Dgas44, and pAet9-N11 can be used to identify genome origin in a polyploid or an interspecific hybrid since they are distributed over all the chromosomes belonging to A genome (Mcintyre et al. 1990; McNeil et al. 1994; Zhang et al. 2004b).
The two types of ribosomal RNA genes (rDNA), 18S-5.8S-26S rDNA and 5S rDNA, have been extensively used as probes for physical mapping in higher plants because these ribosomal RNA genes are arranged in tandem arrays clustered at a few sites. In lower eukaryotes such as yeast and moss, the 5S and 18S-5.8S-26S rRNA genes are in juxtaposition in the same locus, whereas in higher eukaryotes they are organized in separate loci (Appels and Honeycutt 1986). Visualization of 5S and 18S-5.8S-26S rRNA genes by FISH has provided a number of chromosomal markers to elucidate chromosome evolution and species interrelationships, including evolution of polyploid species.
In hexaploid wheat, the six loci of 5S rRNA genes were identified on the short arm of the chromosomes of homoeologous group 1 and 5 (1A, 1B, 1D, 5A, 5B, and 5D) (Mukai et al. 1990), whereas18S-5.8S-26S rDNA loci were mapped on the short arm of 1A, 1B, 6B, and 5D chromosomes, and the long arm of 7D chromosome (Mukai et al. 1991). Double target FISH experiment with 18S-5.8S-26S rDNA and 5S rDNA provides numerous signals on somatic chromosomes. Multicolor FISH combining repetitive DNA probes with rDNA probes also provides a satisfactory number of markers for chromosome identification. 18S 5.8S-26S rRNA genes are associated with the nucleolus organizing region (NOR), and the maximum number of nucleoli at interphase correlates with number of active 18S -5.8S 26S rDNA loci. In polyploid Aegilops, comparisons of FISH patterns of polyploid species with those of diploid progenitors revealed that the natural amphiplasty occurred in polyploid species, in which the active rDNA sites changed to inactive sites or the rDNA sites were deleted during polyploid formation (Yamamoto 1994). In tetraploid species, the U genome mostly suppresses the NOR activity of other genomes. On the other hand, the NOR activity of the D-genome chromosomes is completely suppressed by other genomes. In hexaploid species, all rDNA sites on the third genome remain active, reflecting time lapse after polyploid formation.
Mukai and Apples (1996) were the first to apply in situ polymerase chain reaction (in situ PCR) FISH for mapping plant genes. This method combines the extreme sensitivity of PCR with the cytological location of DNA sequences provided by in situ hybridization.The in situ locations of the rye-specific spacer region were determined on metaphase chromosomes using two pairs of primers designed for rye Nor-R1and rye 5S-Rrna-R1.The sizes of amplificants are 386 bp and 107 bp, respectively.Rye NOR primers generated strong signal in the nucleolus organizing region of rye chromosome 1R and an additional faint signal in the long arm of chromosome 4R. With rye 5S primers, signals appeared on the satellite segment of chromosome 1R and the short arm of chromosome 3R. No signals were detected on the short arm of chromosome 5R which has a previously described locus, 5S-Rrna-R2. The absence of a 5S site is due to sequence differences between the different 5S rDNA lineages. In the same gene family we could detect chromosome specific sequences by selecting primers specific to the chromosome. Thus in situ PCR is expected to be a useful method for amplifying the small region of DNA sequences of specific plant chromosomes and for mapping low copy genes of interest (Mukai and Yamamoto 1998).
Physical mapping of agronomically important genes
Agronomically important genes of wheat are mostly unique or low copy sequences. The information about the exact physical location of agronomically important genes could be useful in breeding programs as well as in understanding the organization of cereal genomes. On human chromosomes, single copy sequences as small as 1 kb could be routinely detected by the standard FISH technique, but in wheat it is difficult to locate 10 kb sequences even when amplification of FISH signals is applied to enhance high resolution FISH efficiency. As a suitable alternative, large genomic clones such as lambda phages, cosmids, BACs, and PACs could be successfully used: the large amount of repeated sequences contained in them would facilitate homologous hybridization, signals detection of which would be suppressed by competitive hybridization with unlabeled C0t -1 DNA or total genomic DNA enabling the unique sequences of interest as expressively detected.
Single or low copy sequences such as genes controlling wheat
grain quality have been routinely mapped on wheat chromosomes using lambda phage
clones containing 10 to 20 kb insert of genomic DNA sequences. Starch consists
of amylose and amylopectin, which differ in the degree of branching; amylopectin
is much more branched than amylose. There are two types of starch branching
enzymes, I (SBEI) and II (SEMI), which control the pattern and degree of branching
in starch in consonance with the various starch syntheses and debranching enzymes.
SBEll is further subdivided into Ila and IIb. Many lambda clones involved in
starch synthesis were isolated from genomic libraries of
Wheat contains at least four classes of starch synthases in the endosperm, granule bound starch synthases I (GBSSI) and starch synthases I, II, and III (SSI, SSII, SSIII). FISH using the lambda phage done G1 was performed to physically locate the genes for SSII on Ae. squarrosa chromosomes (Li et al . 2003). The results show that the genes are located in the middle of the short arm of chromosome 7D (Fig. 3).
Wheat grains can be classified as having either a soft or hard endosperm texture. The hardness of the grain is controlled by a major locus, Ha, on the short arm of chromosome 5D. GSP-I and puroindolines a and b are regarded as good markers for grain hardness and have been proposed as candidate genes for that trait. Two (GSP-1 clones, RG1 and fl-1, were isolated from a soft bread wheat and Ae. squarrosa lambda phage libraries (Turner et al. 1999). As genes for GSP-1 are found on all group 5 chromosomes, it was important to identify the genomic origin of RG1. This was achieved through FISH. There was a clear signal on the end of the short arm of chromosome 5A (Fig. 4). A weak signal was also observed occasionally at the tip of the short arm of chromosome 5B; the sensitivity of the technique was not sufficient to detect the GSP-1 locus on chromosome 5D. On the other hand, attempts to localize Tt-1 to the short arm of chromosome 5D were not successful as the genomic clone included repetitive sequences that hybridized to all chromosomes of wheat even if competitive DNA was added. These results emphasize the differences between the GSP-1 genes in the A and D genomes, with regards to neighboring sequences.
Although the genes for puroindoline-a, puroindoline-b, and GSP-1 are known to be tightly linked to the Ha locus, but the physical location of the genes for puroindoline-a and puroindoline-b has not yet been demonstrated. An extensive study comparing corresponding sequences of diploid and polyploid wheats has unraveled the molecular basis of the evolutionary events observed at the Ha locus (Chantret et al. 2005). FISH was carried out with lambda clones for puroindoline-a and puroindoline-b, but only puroindoline-b localized to the short arm of chromosome 5D (Mukai et al. 1998). FISH was therefore carried out with PCR products covering the gene and sequences approximately 1 kb upstream or downstream of the gene. The total length of the probe was 2.1 kb for puroindoline-a, 2.8 kb for puroindoline-b, and 1.8 kb for GSP-1. With these probes the sequences for these three genes were shown to be physically located at the distal end of the short arm of chromosome 5 of Ae. squarrosa (Turnbull et al. 2003). This showed that there was correspondence between the genetic and physical maps obtained for this chromosome and the tight linkage between the three markers was not merely due to anomalously low recombination.
Two clones having a debranching enzyme gene (a sugary-type isoamylase gene), DBEI and 12, were isolated from Ae. squarrosa BAC and lambda phage libraries, respectively (Rahman et al. 2003), and their chromosomal localizations were confirmed by FISH using PCR amplificants as probes. Consecutive FISH of Ae. squarrosa chromosomes, using DBEI BAC clone probe first (Fig. 5a) and then lambda done 12 probe (Fig. 5b), did not yield any discrete signals. On the other hand, their PCR fragments having only the gene sequences amplified, with no amplification of repetitive sequences, unraveled clear homologous hybridization signals confirming their localization in the proximal region of the short arm of chromosome 7D (Fig. 5c).
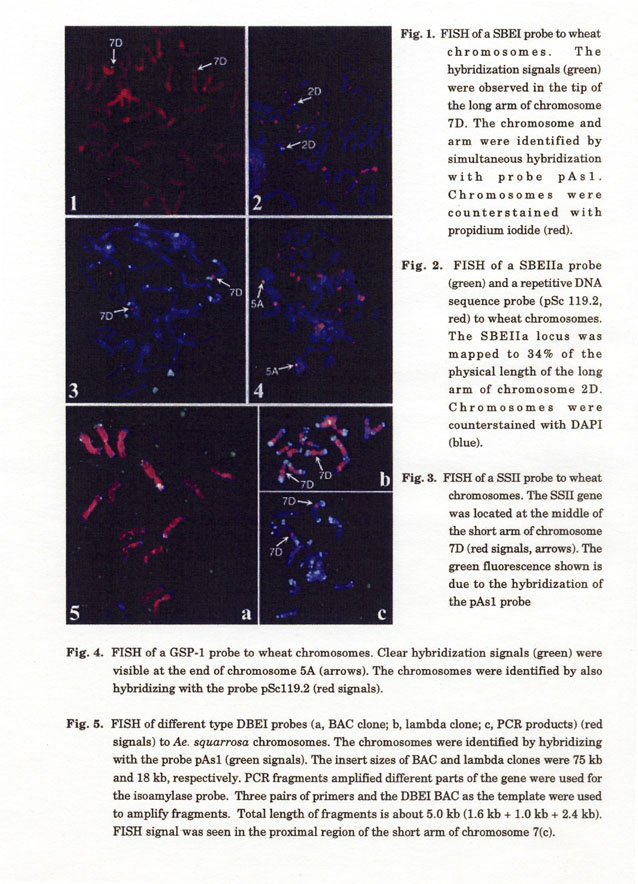
Genome analysis at a glance
The classical methods of genome analysis, like the investigations on chromosome pairing at meiosis and pollen fertility of F1 hybrids have various limitations, e.g. difficulty in obtaining hybrid plants in trees, effect of Ph genes, and so on. The genomic in situ hybridization (GISH) that uses total genomic DNA as the probe is one of the excellent technologies suitable for the visualization of whole genome in the species hybrids and polyploids. GISH has overcome the problems associated with classical methods of genome analysis and successfully discriminated the genomes of many polyploid crops including wheat, and even woody species where it takes long time to observe the chromosome association at meiosis (Raina et al. 1998), Multicolor FISH using total genomic DNA probes is called multicolor GISH and has been applied to genome analysis of polyploid Aegilops and Triticum species and allopolyploids of other taxa (Mukai 1996).
Mukai et al. (1993a) succeeded in simultaneous discrimination of the three genomes in hexaploid wheat using multicolor FISH. Biotin-labeled total genomic DNA of the A-genome progenitor Triticum urartu, digoxigenin-labeled genomic DNA of the D-genome progenitorAe. squarrosa, and unlabeled genomic DNA of one of the possible B-genome progenitors,Ae. speltoides, were used as probes and hybridized to chromosome DNA of T. aestivum cv. Chinese Spring. For detection, two fluorochromes, FITC-conjugated avidin and rhodamine-conjugated anti-digoxigenin, were used. The biotin-labeled probe hybridized to the A -genome chromosomes of wheat could be detected by yellow fluorescence, and the digoxigenin-labeled probe hybridized to the D-genome chromosomes of wheat detected by orange fluorescence. The B-genome chromosome showed faint fluorescence as a result of cross-hybridization of the A- and D-genome probes. As such, the A-, B-, and D-genome chromosomes could be simultaneously detected by their yellow, brown, and orange fluorescence.
Application of BAC-FISH
As bacterial artificial chromosome (BAC) vector can accommodate large-insert genomic DNA, BAC libraries are used most widely at present for analyzing complex plant genomes. Physical mapping by FISH using BAC clones as probes is called BAC-FISH and has been successful in plant species with relatively small genomes, such as sorghum, cotton, rice, Arabidopsis, tomato, potato, and wild sweet potato. The large-scale BAC-FISH analysis in the large genome plants, such as wheat and onion, has been studied at conventional molecular cytogenetical level (Suzuki et al. 2001; Suzuki and Mukai 2004; Zhang et al. 2004a, 2004b).
Randomly selected 202 BAC clones of Ae. squarrosa were applied to FISH analysis on chromosomes of common wheat in the absence of C0t -1 genomic DNA (Suzuki and Mukai 2004). They did not use C0t -1 genomic DNA as competitor in the BAC-FISH screening because cytogenetic markers detecting FISH signal without competitive genomic DNA are useful in simultaneous detection of the target loci and the markers by multicolor FISH. Most of the clones (163 clones) were hybridized with entire chromosomes as dispersed signals that maybe due to the repetitive sequences dispersed on the wheat genome. These clones were classified into three types based on the signal pattern. Signals of the 91 clones were detected uniformly on the chromosomes. On the other hand, mottled signals on the chromosomes were detected for 45 clones. Interestingly, signals of 27 clones are more specific to the D genome; these clones might contain D-genome specific repetitive sequences. Localized signals were obtained in 19 clones. The signals of 10 of the 19 clones showed centromeric localization, that of two clones showed sub-telomeric localization, and banded signals were obtained in the remaining seven clones. The clones showing the localized signals on centromeres or subtelomeres are useful to characterize the location of the centromeres and telomeres during the mitosis and meiosis. In the clone showing banded signals, one clone showed the signals on nine regions of five chromosomes; two separate loci on different arms of chromosome 1D, each set of two closely linked loci on chromosomes 4D, 5D and 6D, and one locus on chromosome 2D. However, there are no clones detecting a single locus by the BAC-FISH, suggesting that the FISH signals on the specific region are hard to be obtained in this method.
Zhang et al. (2004a, 2004b) selected 56 RFLP locus-specific BAC clones from libraries of Ae. squarrosa and A-genome diploid T. monococcum. Different types of repeated sequences were identified using BAC-FISH: two BAC clones gave FISH patterns similar to the repetitive DNA family pSc119; one BAC clone gave a FISH pattern similar to pAs1; one BAC clone hybridized to the centromeric regions of wheat; one BAC clone hybridized to five D-genome chromosomes pairs in wheat; and four BAC clones hybridized only to a proximal region in the long arm of chromosome 4A of hexaploid wheat. These clones can be used for chromosome identification. In addition to the above tandem repeats, three dispersed repeats were identified: the BAC clone 676D4 from the T. monococcum library preferentially hybridized to the A-genome chromosomes of wheat and two BAC clones, 9110 and 9M13, from the Ae. squarrosa library hybridized to the D-genome chromosomes. These clones are useful in simultaneously discriminating the three different genomes in hexaploid wheat, and in identifying intergenomic translocations in wheat. Sequencing data show that all these repeats are transposable elements, indicating the important role of retrotransposons in the genome evolution of wheat.
FISH analysis on DNA fibers in Triticeae
With the aid of FISH techniques, it has now been possible to physically visualize genes and DNA sequences under a microscope on extended DNA fibers (EDFs) from interphase nuclei. In wheat EDF fibers could be obtained even from metaphase chromosomes (Lavania et al. 2003). FISH on extended DNA fibers is a useful tool for determining the sizes of target DNA sequences, the order of genes or clones and their distances in a large chromosomal region (Fransz et al. 1996; Suzuki et al. 2004). Currently, fiber FISH has indicated the potential for tracing the target sequences with lengths of up to 2 Mb on single EDFs, a spatial resolution of 1 kb between adjacent targets, and detection sensitivity of a target of as small as 700 kb in Triticeae plants (Yamamoto and Mukai 1998; Fukui et al. 2001).DNA probes of the 18S-5.8S-26S rRNA and 5S rRNA genes in common wheat (Chinese Spring) were used in fiber FISH experiments to estimate the sizes of these multigene families (Yamamoto and Mukai 1998). The hybridization signals of the 18S-5.8S-26S rRNA genes in EDFs were traced with lengths of up to 625 μm. Taking the value of the B-form DNA equivalent to 3.27 kb for 1 μm (Fransz et al. 1996), the microscopical length of the 18S-5.8S-26S rRNA signals was estimated to correspond to a molecular size of 2 An. In Chinese Spring wheat, there are 9,100 copies of the gene per haploid genome (Flavell and O'Dell 1979). About 100 - 5,000 copies are present at nucleolar organizing region of each satellited chromosome. The length of the repeating unit of rDNA is 8.9 kb. The length of 2 Mb DNA corresponds to 225 copies of one rDNA unit.
The 5S RNA genes are independent of the rDNA and are organized in tandem repeats. In wheat, there are two lineages for the 5S RNA multigenes having repeats of different lengths of 500 bp and 400 bp. There are two types of signals: continuous and discontinuous fluorescent strings. Three to ten discontinuous strings within the 50-150 kb size range were lined up in tandem (a total of 300 to 950 kb). On the other hand, continuous signals were observed as fluorescent strings with lengths of 380 to 1,200 kb. The maximum size (1,200 kb) of 5S rRNA gene cluster is estimated to be 2,400 to 3,000 copies.
FISH analysis of the centromeric region on EDFs visualized that
the Ty3/gypsy retrotransposon-like elements are tandemly repeated in
wheat centromeres (Fukui et al.2001). The centromeric retrotransposon-like
elements consist of highly conserved integrase and CCS1 sequences. Fluorescent
signals of each sequence were clearly detected as a dot although the integrase
and CCS1 fragments used as probes were about 800 bp and 1.7 kb, respectively.
The average interval between the integrase sequences was 50-65 kb.
High-resolution mapping of secalin-1 (Sec-1) locus
has been performed by FISH to extended DNA fibers of rye, employing DNA probes
of lambda phage clones containing the w-secalin gene (Yamamoto and Mukai 1998,
2005). The fluorescent signals on extended DNA fibers of rye revealed continuous
strings of 45 μm, corresponding to the size of 147 kb DNA. To determine
the copy number of Sec-1 locus on DNA fibers, a 1.2 kb fragment including the
entire coding region of the w-secalin gene and a 1.0 kb fragment of the promoter
region were amplified by PCR as probes for another fiber FISH. Physical position
of these sequences was visualized as alternating fluorescent spots by multicolor
in situ hybridization. Alternating signals of two DNA probes reflected the tandem
repeated organization of Sec-1 locus having 15 copies of the gene.
The findings based on fiber FISH analysis support the contention that the w-secalin
genes are arranged in a head to tail fashion separated by 8 kb of spacer sequences
with a total length of 145 kb (Clarke et al. 1996). In transgenic plants,
fiber FISH can physically map the transgenes directly on extended DNA fibers.
This method is complementary to PCR, Southern blot and sequence analyses (Jackson
et al. 2001; Nakano et al. 2005) and is discussed later in
this article.
Recently molecular combing has made progress as a new technique
to map directly cloned DNA sequences on individual stretched DNA molecules.
Application of molecular combing FISH facilitates determining the structure
of a clone, copy number of genes, and the order of genes and specific sequences.
A small fragment (2.5 kb) of the starch branching enzyme I gene (Sbe-1)
was mapped on DNA molecules of a BAC clone (SBEI) by FISH (Suzuki et al.
2003). Rahman et al. (1997) presumed existence of 4-5 copies of the
Sbe-1 gene in the Ae. squarrosa genome, and mapped the genes
on the short arm of chromosome 7D. Three clear hybridization signals within
30 kb of each other were observed on the majority of the insert fragments of
BAC SBEI molecules (Fig. 6). The results indicate that DNA fragments as small
as 2.5 kb can be visualized on BAC molecules using this technique and there
exist at least three copies of the gene in BAC SBEI.
The orientation of the seed storage protein and grain-hardness
genes was analyzed on wheat BAC DNA molecules (circular and linear types) using
BAC clones forming a contig (Yamamoto and Mukai 2004). The accurate determination
of the overlap between the adjacent clones has also been elucidated. This saves
time and labor in analysis and provides useful information for construction
of BAC contig. Thus, the visual information by high resolution mapping will
be useful for detailed analysis of structure and organization of large insert
clones.
Application of FISH to wheat breeding
Limitation of GISH was reported in routine screening for alien introgression in wheat (Lukaszewski et al. 2005). GISH technique failed to reveal the presence of some distally located breakpoints. The limit of resolution was at least 3.5 cM (7%) of the relative genetic lengths of chromosome arms in configurations with proximal rye and terminal wheat segments when rye DNA was used as a probe. When wheat DNA was used as a probe, a terminal wheat segment estimated to be 1.6 cM in length could not be visualized.
The most important task in current plant breeding research is to introduce a set of agronomically useful genes in a given crop across the barriers of taxonomy and reproductive isolation. It is therefore, necessary in practical plant genomics that suitable transformation systems are established that facilitate introduction / integration of large genomic fragments loaded with several desired gene functions from a distant gene source into the recipient crop beyond the barrier of reproductive isolation.
FISH could complement such efforts by supplementing information on size of genomic insert and its possible integration sites that will have value in understanding amenability of genomic insertion and its acceptability and expression. This is very important in practical plant breeding since very little is known about where transgenes land, and what effect do they impart onto the recipient organism in terms of stability and expression.
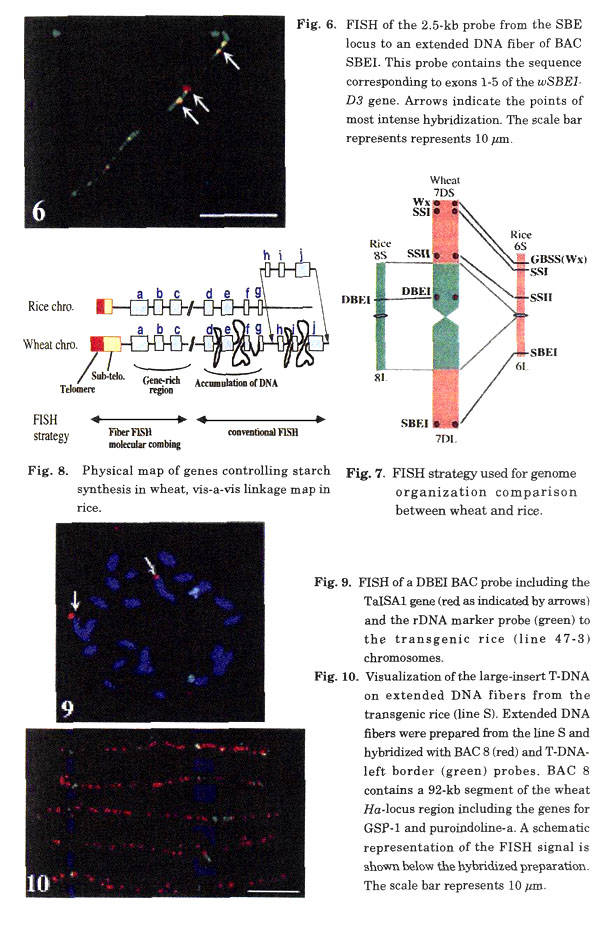
Synteny between wheat and rice genes
Genomics concerned with the systematic molecular characterization of whole genome is important for obtaining an overview of the genome organization and to provide the basic information for isolating specific genes or DNA sequences. At present various projects in many higher plants are at various stages of sequencing and genome analysis.
Genomic sequencing provides information about global genome structure and organization, regulatory regions, transposable elements, and non-coding sequences. Comparative genomics is the study of the similarities and differences in structure and function of genetic information across taxa at the DNA level using molecular tools (Paterson et al. 2000). In cereals, covering most of the world's food and feed crops, a consensus map of 12 grass genomes is now available and it represents the chromosome segment of each genome relative to rice on the basis of the mapping of anchor DNA markers (Gale and Devos 1998; Devos and Gale 2000). However, the comparison of gene location along chromosomes has been mainly conducted in terms of genetic map, and not physical map. Therefore we need to determine the accurate position of genes of interest by physical mapping by FISH.
Cereal genomes are highly variable in size, ranging from 430 Mb in rice to 16,700 Mb in hexaploid wheat. Comparative genomic analysis at the genetic-map level has shown extensive conservation of the gene order between the different grass genomes in many chromosomal regions. However, little is known about the gene organization in grass genomes at the microlevel. Comparison of gene-coding regions between cereals showed that the distance between the genes is correlated with the genome size. In comparison to rice, large amount of DNA is lying in between the genes on wheat chromosomes (Table 1, Fig. 7). On the other hand, rice and wheat genomes contain regions that are highly enriched in genes with very little or no repetitive DNA. Telomeric regions of both genomes revealed a density of one gene per 5~15 kb, very similar to the gene density in Arabidopsis thaliana. For example, at least eight genes were found in 94 kb DNA of the wheat Ha locus region (Chantret et al. 2005). The comparison of the gene organization suggested various genome rearrangements during the evolution of the different grass species.
We have mapped several genes on wheat chromosomes by FISH involved in starch synthesis and compared the position of these genes on linkage map of rice (Li et al. 2003). The results show that gene synteny was well conserved in the wheat and rice genomes, including even in large blocks often comprising entire chromosome, despite huge differences in the genome size (Fig. 8).
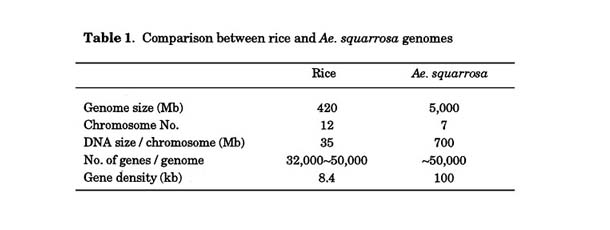
Trans-introduction of large wheat genomic fragments into rice
In order to expand genetic variability in rice, we have attempted to introduce the large fragments of genomic DNA containing agronomically important genes of wheat into rice. We have successively introduced huge DNA fragments via BAC clones from wheat into rice by 'genome fusion' method. In order to achieve this, as a first step, we developed a system that facilitates efficient introduction of huge mass of wheat genomic DNA into rice. Our prime goal is to realize bread making and udon noodles features in rice powder, for which we have focused our efforts on the introduction of three types of gene clusters from wheat into rice, i.e. starch synthesis-related genes, grain hardness-related genes and prolamin-related genes. It is surmised that full realization of targeted objectives would result in expanded rice consumption, necessitating increased rice cultivation, facilitating environmental conservation, and also help import-substitution for wheat flour.
The 75 kb of Ae. squarrosa genome insert containing the wheat isoamylase 1 (TaISA1) gene was transformed to rice by Agrobacterium -mediated transformation method using BAC vector (Kubo et al. 2005). The presence of the transgenes in regenerated plants was confirmed by PCR analysis using primers for a part of the gene. Then, to detect wheat DNA fragments cytologically, we carried out FISH experiments using the original large DNA fragment as a probe in several lines of transgenic rice. Two hybridization signals were observed in metaphase chromosomes in the homozygous T2 plants of each line (Fig. 9). Most signals appeared at the terminal or distal regions of rice chromosomes. The fragments of wheat genome DNA were stably transmitted to offspring and the transgenes were expressed in rice.
Little information is available about the organization and stability of large fragments of foreign DNA in transgenic plants. We also introduced a 92-kb DNA fragment of the wheat Ha-locus region into rice by Agrobacterium -mediated transformation (Nakano et al. 2005). The structures of the T-DNA in four independent transgenic lines were visualized by fluorescence in situ hybridization on extended DNA fibers. A long and contiguous red signal with two short green signals was observed on the DNA fiber (Fig. 10). The length of the integrated region was estimated to be 140 kb. The possible integration events are summarized as follows: two copies of the BAC 8 construct have been integrated in inverted orientation, a deletion has occurred in one of the copies, and short segments of T-DNA and insert DNA have been duplicated and transferred to the terminal region. Rearrangements of the large-insert T-DNA, involving duplication, deletion and insertion, had occurred in all four lines. This result suggested that the large T-DNAs integrated by Agrobacterium -mediated transformation are subject to be reorganized in transgenic rice plants. The integration of intact T-DNA should, therefore, be routinely confirmed by molecular cytogenetic analysis in the. case of transgenic plants transformed with large transgenes using Agrobacterium.
Concluding remarks
Molecular approaches to wheat cytogenetics have generated useful knowledge base on alien relatives and genomic resources of valuable gene pool, enriching wheat genomics to complement breeding research. Isolation and molecular characterization of multiple repetitive sequences, each representing a substantial fraction of the genome followed by their physical localization has provided a novel top-down chromosomal approach to complement bottom-up DNA markers to facilitate clone based genome analysis to visualize genomic organization, chromosome structure and landmarks for looking at genes, their clustering and orientation. This has far reaching repercussions on transfer and stable integration of transgenes across the taxonomic barriers. Availability of genome fusion technology opens new vistas on transfer of fully functional genes to realize novel genomic complementation. Such developments have opened immense possibilities in plant genomics research in general and wheat genomics in particular. To make best use of molecular cytogenetics approaches to wheat research in the 21st century, it would be most desirable to focus our efforts on identification of agronomic / qualitative and developmentally useful genes in wheat, its alien relatives as well as wild resources, and assign them to their physical linkage groups with targeted objectives of their transgene integration for value addition. With the current state of technological advancement and future possibilities, this could be conveniently done following molecular cytogenetic analysis (1) from chromosome level to DNA fiber level; (2) from individual analysis to comparative analysis; (3) from structural analysis to functional analysis; (4) from segregated comprehension to integrated comprehension; and (5) from basic study to applied study, following an integrated multidisciplinary approach.
Acknowledgements
I consider it my pleasant duty to record the support of my associates in the
laboratory, Drs. Maki Yamamoto, Sadiquer Rahman and Go Suzuki, and other young
researchers over a period of time, who contributed and sustained our research
efforts in understanding molecular cytogenetics of wheat.
References
Appels R and Honeycutt RL (1986) rDNA evolution over a billion years. In: Dutta
SK (ed.) DNA Systematics. CRC Press, Boca Raton, USA, pp. 81-125.
Appels R, Gerlach WL, Dennis ES, Swift H and Peacock WJ (1980) Molecular and
chromosomal organization of DNA sequences coding for the ribosomal RNAs in cereals.
Chromosoma 78: 293-311.
Bedbrook JR, Jones J, O'Dell M, Thompson RD and Flavell RB (1980) A molecular
description of telomeric heterochromatin in Secale species. Cell 19:
545-560.
Chantret N, Salse J, Sabot F, Rahman S, Bellec A, Laubin B, Dubois I, Dossat
C, Sourdille P, Joudrier P, Gautier MF, Cattolico L, Beckert M, Aubourg S, Weissenbach
J, Caboche M, Bernard M, Leroy P and Chaloub B (2005) Molecular basis of evolutionary
events that shaped the hardness locus in diploid and polyploid wheat species
(Triticum and Aegilops).Plant Cell 17: 1033-1045.
Clarke BC, Mukai Y and Appels R (1996) The Sec-1 locus on the short
arm of chromosome 1R of rye (Secale cereale). Chromosoma 105: 269-275.
Devos KM and Gale MD (2000) Genome relationships: The grass model in current
research. Plant Cell 12: 637-646.
Endo TR, Yamamoto M and Mukai Y (1994) Structural changes of rye chromosome
1R induced by a gametocidal chromosome. Japan J Genet 69: 13-19.
Flavell RB and O'Dell (1979) The genetic control of nucleous formation in wheat.
Chromosoma 71: 135-152.
Fransz PF, Alonso-Blanco C, Liharska TB, Peeters AJM, Zabel P and de Jong JH (1996) High-resolution physical mapping in Arabidopsis thaliana and tomato by fluorescence in situo? hybridization to extended DNA fibers. Plant J 9: 421-430.
Freibe B, Hatchett JH, Gill BS , Mukai Y and Sebesta EE (1991) Transfer of Hessian fly resistance from rye to wheat via radiation-induced terminal and intercalary chromosomal translocations. Theor Appl Genet 83: 775.782.
Freibe B, Zhang P, Linc G and Gill BS (2005) Robertsonian translocations in wheat arise by centric misdivision of univalents at anaphase I and rejoining of broken centromeres during interkinesis of meiosis. II. Cytogenet Genome Res 109: 293-297.
Fukui K-I, Suzuki G, Lagudah ES, Rahman R, Appels R, Yamainoto M and Mukai Y (2001) Physical arrangement of retrotransposon-related repeats in centromeric regions of wheat. Plant Cell Physiol 42:189-196.
Gale MD and Devos KM (1998) Plant comparative genetics after 10 years. Science 282: 656-659.
Gerlach WL and Peacock WJ (1980) Chromosomal locations of highly repeated DNA sequences in wheat. Heredity 44: 269-276.
Jackson SA, Zhang P, Chen WP, Philips RL, Friebe B, Muthukrishnan S and GM BS (2001) High resolution structural analysis of biolistic transgene integration into the genome of wheat. Theor Appl Genet 103: 56-62.
Kubo A, Rahman S, Utsumi Y, Li Z, Mukai Y, Yamamoto M, Ugaki M, Harada K, Satoh H, Konik-Rose C, Morell M and Nakaniura Y (2005) Complementation of sugary-1 phenotype in rice endosperm with the wheat isoamylasel gene supports a direct role for isoamylasel in amylopectin biosynthesis. Plant Physiol 137: 43-56.
Langer-Safer PR, Levine M and Ward DC (1982) Immunocytological method for mapping genes on Drosophila polytene chromosomes. Proc Natl Acad Sci USA 79:4381-4385.
Lavania UC, Yamamoto M and Mukai Y (2003) Extended chromatin and DNA fibers from active plant nuclei for high resolution FISH. J Histochem Cytochem 51: 1249-1253.
Li Z, Sun F, Xu S, Chu X, MUkai Y, Yamamoto M, All S, Rampling L, Kosar-Hashemi B, Rahman S and Morell MX (2003) The structural organisation of the genes encoding class II starch synthase of wheat and barley and the evolution of the genes encoding starch syuthases in plants. Fund Integr Genomics 3:76-85.
Lukaszewski AJ, Lapinski B and Rybka K (2005) Limitations of in situ hybridization with total genomic DNA in routine screening for alien introgrssions in wheat. Cytogenet Genome Res 109: 373-377.
Masoudi-Nejad A, Nasuda S, Mcintosh RA and Endo TR (2002) Transfer of rye chromosome segments to wheat by a gametocidal system. Chromosome Res 10: 349-357.
McIntyre CL, Pereira S, Moran LB and Appels R (1990) New Secale cereale (rye) DNA derivatives for the detection of rye chromosome segments in wheat. Genome 33: 635-640.
McNeil D, Lagudah ES, Hohmann U and Appels R (1994) Amplification of DNA sequences in wheat and its relatives: the Dgas44 and R350 families of repetitive sequences. Genome 37: 320-327.
Mukai Y (1996) Multicolor fluorescence in situ hybridization: a new tool for genome analysis. In: Jauhar PP (ed.) Methods of Genome Analysis in Plants, CRC Press, Boca Raton, USA, pp. 181-192.
Mukai Y (2004) Fluorescence in situ hybridization, In: Goodman RM (ed.) Encyclopedia of Plant and Crop Science, Marcel Dekker, New York, USA, pp. 468-471.
Mukai Y and Appels R (1996) Direct chromosome mapping of plant genes by in situ polymerase chain reaction (in situ PCR). Chromosome Res 4: 401-404.
Mukai Y and Yamamoto M (1998) Application of multicolor fluorescence in situ hybridization to plant genome analysis. In: Gupta PK (ed.) Genetics and Biotechnology in Crop Improvement, Rastogi Pub, Meerut, India, pp. 14-23.
Mukai Y, Endo TR and Gill BS (1990) Physical mapping of the 5S rRNA multigene family in common wheat. J Heredity 81: 290-295.
Mukai Y, Endo TR and Gill BS (1991) Physical mapping of the 18S.26S rRNA multigene family in common wheat: Identification of a new locus. Chromosoma 100: 71-78.
Mukai Y, Friebe B and Gill BS (1992) Comparison of C-banding patterns and in situ hybridization sites using highly repetitive and total genomic rye DNA probes of 'Imperial' rye chromosomes added to 'Chinese Spring' wheat. Japan J Genet 67: 71-83.
Mukai Y, Nakahara Y and Yamainoto M (1993a) Simultaneous discrimination of the three genomes in hexaploid wheat by multicolor fluorescence in situ hybridization using total genomic and highly repeated DNA probes. Genome 36: 489-494.
Mukai Y, Friebe B, Hatchett JH, Yamamoto M and Gill BS (1993b) Molecular cytogenetic analysis of radiation-induced wheat-rye terminal and intercalary chromosomal translocations and the detection of rye chromatin specifying resistance to Hessian fly. Chromosoma 102: 88-95.
Mukai Y, Rahman S, Yamamoto M, Okamoto M, Turner M, Li Z, Morell M and Appels R (1998) Physical mapping of genes controlling wheat grain quality by fluorescence in situ hybridization. Proc 9th hit Wheat Genet Symp, Saskatoon, 4: 218-221.
Nakano A, Suzuki G, Yamamoto M, Turnbull K, Rahman S and Mukai Y (2005) Integration and organization of large-insert T-DNA in rice: visualization of rearrangements of large transgenes. Mol Gen Genomics 273:123-129.
Paterson AH, Bowers JE, Burow MD, Draye X, Elsik CG, Jiang C-X, Kaster CS, Lan T-H, Lin Y-R, Ming R and Wright RJ (2000) Comparative genomics of plant chromosomes. Plant Cell 12: 1523-1539.
Pedersen C and Langridge P (1997) Identification of the entire chromosome complement of bread wheat by two-color FISH. Genome 40: 589-593.
Rahman S, Abrahains S, Abbott D, Mukai Y, Samuel M, Morell M and Appels R (1997) A complex arrangement of genes at a starch branching enzyme I locus in the D genome donor of wheat. Genome 40: 465-474.
Rahman S, Regina A, Li Z, Mukai Y, Yamamoto M, Kosar-Hashemi B, Abrahams S and Morell MK (2001) Comparison of starch-branching enzyme genes reveals evolutionary relationships among isoforms. Chracterisation of a gene for starch-branching enzyme IIa from the wheat D genome donor Aegilops tauschii. Plant Physiol 125: 1314-1324.
Rahman S, Nakamura Y, Li Z, Clarke B, Fujita N, Mukai Y, Yamamoto M, Regina A, Tan T, Kawasaki S and Morell M (2003) The sugary-type isoamylase gene from rice and Aegilops tauschii: characterization and comparison with maize and Arabidopsis. Genome 46: 496-506.
Raina SN, Mukai Y and Yamamoto M (1998) In situ hybridization identifies the diploid progenitor species of Coffea arabica (Rubiaceae). Theor Appl Genet 97: 1204-1209.
Rayburn AL and Gill BS (1985) Use of biotin-labeled probes to map specific DNA sequences on wheat chromosomes. J Hered 76: 78-81.
Rayburn AL and Gill BS (1986) Molecular analysis of the D-genome chromosomes of wheat. J Hered 77: 253-255.
Suzuki G, Ura A, Saito N, Do GS, Seo BB, Yamamoto M and Mukai Y (2001) BAC FISH analysis in Allium cepa. Genes Genet Syst 76: 251-255.
Suzuki G, Moriyama M, Fujioka K, Yamamoto M, Subranianyam NC, Li Z, Appels R, Morell M, Mukai Y and Rahman S (2003) The starch branching enzyme I locus from Aegilops tauschii, the donor of the D genome to wheat. Funct Integr Genomics 3: 69-75.
Suzuki G and Mn kai Y (2004) Plant BAC libraries as tools for molecular cytogenetics. In: Williams CR (ed.) Focus on Genome Research, Nova Science Publishers, New York, USA, pp. 195-210.
Suzuki G, Tanaka S, Yamamoto M, Tomita RN, Kowyama Y and Mukai Y (2004) Visualization of the S-locus region in Ipomoea trifida: toward positional cloning of self-incompatibility genes. Chromosome Res 12:475-481.
Tsujimoto H, Mukai Y, Akagawa K, Nagaki K, Fujigaki J, Yamamoto M and Sasakuma T (1997)Identification of barley chromosomes by repetitive sequences: Conservative distribution of Afa-family repetitive sequences on the chromosomes of barley and wheat. Genes Genet Syst 72:303-309.
Turnbull K-M, Turner M, Mukai Y, Yamamoto M, Morell MK, Appels R and Rahman S (2003) The organization of genes tightly linked to the Ha locus in Aegilops tauschii the D genome donor to wheat. Genome 46:330-338.
Turner M, Mukai Y, Leroy P, Charef B, Appels R and Rahman S (1999) The Ha locus of a polymorphic region for tracing grain hardness in crosses. Genome 42: 1242-1250.
Yamamoto M (1994) Molecular-cytogenetic analysis of the rDNA region in Triticum and Aegilops. PhD thesis, Kyoto University, Japan, pp. 248.
Yamamoto M and Mukai Y (1989) Application of fluorescence in situ hybridization to molecular cytogenetics of wheat. Wheat Inf Serv 69: 30-32.
Yamamoto M and Mukai Y (1998) High-resolution mapping in wheat and rye by FISH on extended DNA fibers. Proc IX Int Wheat Genet Symp Saskatoon 1: 12-16.
Yamamoto M and Mukai Y (2004) Molecular combing for high-resolution mapping and its applications. In: Fukui K and Xin Z (eds.) Advance in Chromosome Sciences, CAST Press, Beijing, China, pp. 492-495.
Yamamoto M and Mukai Y (2005) High-resolution physical mapping of the secalin-1 locus of rye on extended DNA fibers. Cytogenet Genome Res 109: 79-82.
Zhang P, Li W, Fellers J, Friebe B and Gill BS (2004a) BAC-FISH in wheat identifies chromosome landmarks consisting of different types of transposable elements. Chromosoma 112: 288-299.
Zhang P, Li W, Friebe B and Gill BS (2004b) Simultaneous painting of three genomes in hexaploid wheat by BAC-FISH. Genome 47: 979-987.