Memorial Issue, Wheat Information Service No.100
The wheat super domestication gene Q
J. D. Fans1, K J. Simons1'2, Z. Zhang1'3 and B. S. Gill2
1USDA-ARS Cereal Crops Research Unit, Northern Crop Science Laboratory,
1307 18th Street North, Fargo, ND 58105, USA
2Department of Plant Pathology, Throckmorton Plant Sciences Center,
Kansas State University, Manhattan, KS 66506, USA
3Department of Plant Sciences, Loftsgard Hall, North Dakota State
University, Fargo, ND 58105, USA
Corresponding author: Justin D. Faris
USDA-ARS Cereal Crops Research Unit, Northern Crop Science Laboratory, 1307
18th Street North, Fargo, ND 58105, USA
Email address: justin.faris@ndsu.edu
Summary
The Q gene is largely responsible for the domestication and widespread cultivation of macaroni and bread wheat primarily because it confers the free-threshing character. It also pleiotropically influences many other domestication-related traits such as glume shape and tenacity, rachis fragility, plant height, spike length, and ear emergence time. For nearly a century, scientists have been intrigued by the Q gene and its pleiotropic nature. Here, we provide a summary of early investigations and discoveries regarding Q and its role in floral morphology and domestication, followed by an overview of our work to clone and characterize Q. Fine-mapping, chromosome walking, and DNA sequence analysis of knockout mutants allowed us to verify that Q is a member of the AP2 class of transcription factors. Analysis of transgenic plants confirmed the dosage and pleiotropic effects of Q on characters such as glume shape and tenacity, rachis fragility, and plant height. The Q and q alleles harbor structural differences, and Q is more abundantly transcribed than q. The q allele is the more primitive, and Q arose only once most likely in a tetraploid, which upon hybridization with Aegilops tauschii produced Triticum aestivum, and from those wheats originated the world's macaroni and bread wheat. Future work will focus on the functional analysis of Q and elucidating associated genes and pathways. Unique methods of improving wheat or domesticating new crops may result from further exploitation of Q, one of the most important genes in the rise of modern civilization.
Key words: speltoid, free threshing, positional cloning, domestication
Introduction
Our distant ancestors were hunter-gatherers for over a million years and relied exclusively on the hunting of wild animals and gathering of wild plants for their food. Then, about 7,000-12,000 years ago, the domestication of wild forms launched an agricultural revolution and the dawn of modern human civilization. Today, the three major cereal crops, wheat, rice, and maize, provide most of the calories consumed by humans. The conversion of wild forms of these crops into modern domesticates resulted from certain genetic mutations that allowed them to be more easily cultivated by early farmers. Mutations that gave rise to traits such as soft glumes, tough rachis, and the free-threshing character were essential for domestication. These and other changes resulted in the conversion of small-seeded wild grasses with natural seed dispersal mechanisms into large-grained non-shattering forms that relied on farmers to harvest the grain.
Wheat exists at three ploidy levels: diploid, tetraploid, and hexaploid. Wild diploid and tetraploid wheat species are widely distributed in the Middle East. Diploid einkorn wheat was domesticated first from Triticum boeoticum (2n = 2x = 14, AA genomes) in SE Turkey about 11,000 years age (Heun et al. 1997). The tetraploid T. turgidum ssp. dicoccum (2n = 4x = 28, AABB genomes), or emmer wheat, was domesticated next from ssp. dicoccoides (wild emmer) (Ozkan et al. 2002). Einkorn and emmer wheat should be considered as only semi-domesticated because they produced hulled seed. Wild forms of hexaploid wheats (T. aestivum, 2n = 6x = 42, AABBDD genomes) do not exist, but primitive forms arose about 8,000 years ago (Huang et al. 2002) through hybridization of an AB-tetraploid with Aegilops tauschii (2n = 2x = 14, DD genomes) (Kihara 1944; McFadden and Sears 1946).
The two modern domesticated wheats that are widely grown today include T. turgidum ssp. durum, or macaroni wheat, and T. aestivum ssp. aestivum, or bread wheat. Because the Q gene confers the free-threshing character and influences a repertoire of other domestication-related traits, it is largely responsible for their widespread cultivation. The wild relatives and primitive wheats have the q allele, which confers a speltoid spike characterized by a spear-shaped head with elongated rachis and non free-threshing seed. Therefore, the mutation that gave rise to Q had a profound effect on agriculture and human civilization because it allowed early farmers to efficiently harvest their grain on a larger scale, and this led to the rapid spread of wheat cultivation over the world. Pioneering wheat geneticists realized this fact in the early 1900's, and Q, often referred to as 'the super gene' in the early literature, has been the subject of great theoretical interest and scientific study ever since. From a practical point of view, Q is arguably one of the most important agricultural genes in human history, and today it is Q that essentially allows wheat to be mechanically harvested on a massive scale.
In this article, we review the scientific history of the Q locus and early discoveries regarding the nature of Q. This is followed by an overview of our work on the cloning and characterization of the gene and how the structure of Q reveals insights into the origin of the cultivated macaroni and bread wheats. Finally, we discuss some future prospects for defining the functional nature of the super gene, Q.
History of Q
Identification of Q: The variant speltoid phenotype was first observed and reported by Nilsson-Ehle (1917, 1920). The speltoid character (Fig. 1) was found to be controlled by a factor(s) on a pair of chromosomes designated by the symbol C by Winge (1929) and later 5A by Sears (1958). Watkins (1927, 1928, 1930, 1940) suggested that the gene k controlled the nonkeeled glume and speltoid suppression characters, while a different gene, q, governed the squareheaded phenotype. These two genes were thought to be linked on the same chromosome arm. Later, MacKey (1954) showed that K and q were actually the same gene. Therefore, variations in glume keeledness as well as speltoid suppression and squareheadedness were considered to be controlled by a single gene (Unrau et al. 1950), which was given the designation Q (MacKey 1954).
The fact that glume keeledness and speltoid suppression were controlled by the same gene led a number of early researchers to investigate the genetics of speltoid suppression by observing only glume shape. Watkins (1928, 1940) assumed that because all tetraploid T. turgidum spp. had keeled glumes, they must carry K. Later, when MacKey (1954) determined that K and q were the same gene, it was then assumed that these tetraploids and any genotype with keeled glumes all carried the q allele. However, Muramatsu (1963, 1979, 1985, 1986) showed that glume keeledness was largely dependent on genetic background and modifier genes at alternate loci. He conclusively demonstrated that a number of keeled glume genotypes possessed Q .
Characters influenced by Q : In addition to glume shape, speltoid suppression, and spike density (squareheadedness), the expression of Q affects other characters. However, much like glume keeledness, each characteristic is subjected to modification by other genes depending on the genetic background. Tsunewaki and Jenkins (1961) used monosomic analysis to show that genes for maturity and plant height are located on chromosome 5A, and later it was shown that these traits are influenced by the Q locus (Muramatsu 1963, Kato et al. 1999, 2003). Singh (1969) reported that radiation induced speltoid mutants showed obvious differences in spike length, spikelet size, maturity, seed fertility, glume tenacity, and threshability. Glume tenacity and rachis fragility were found to be influenced by the Q locus in a number of additional studies (Leighty and Boshnakian 1921; Singh et al. 1957; MacKey 1966; Muramatsu 1979, 1985; Jantasuriyarat et al. 2004). Kerber and Rowland (1974) showed that the TOUGH GLUME (Tg) gene on chromosome 2D in Ae. tauschii is epistatic to Q because synthetic hexaploids derived from a Q -tetraploid and Ae. tauschii are speltoid and non free-threshing. Therefore, a genotype Q Q tgtg is needed to confer the free-threshing character.
Q vs. q: It is the Q allele that makes ssp. aestivum different from ssp. spelta and speltoid mutants, which have a deficiency for the Q locus (Smith et al. 1949; MacKey 1954). Differences between the ssp. spelta and speltoid mutants are slight and probably due to the presence of the q allele in ssp. spelta, but modifier genes at other loci are involved (MacKey 1954). Muramatsu (1963) claimed that ssp. spelta is actually more speltoid than the speltoid mutants, not because of the situation of the Q locus, but in spite of it. It is the genotypic background that makes ssp. spelta more extremely spelted than speltoid mutants, but actually, q in ssp. spelta has less of a spelting effect than does a deficiency for the locus.


In general, Q is considered incompletely dominant to q, and plants with the genotype Qq have a spike morphology that is intermediate to speltoid and squareheaded. However, the genetic background tends to affect the perceived gene action of traits known to be influenced by Q. Muramatsu (1963) stated that, in any given hexaploid background with sufficiently high dosage of Q or q, all the characters controlled by Q will be of "aestivum" type, while at lower dosage they will resemble "spelta". At intermediate dosages some characters may be of aestivum type while others will be more like spelta. Therefore, in heterozygotes, which have intermediate dosage, a particular F1 maybe like aestivum in one respect but more like spelta in others. In Chinese Spring, squareheadedness is fully recessive and the glume and rachis characters are incompletely dominant (Muramatsu 1963). In this case, q might be considered dominant over Q, but the average degree of dominancy may favor Q in different backgrounds.
Dosage effects: The dosage effects of Q and q on spike compactness and other traits has been well established. Plants that are monosomic, disomic, trisomic, and tetrasonuc for chromosome 5A harboring the Q allele have phenotypes that are speltoid, normal (square), subcompactoid, and compactoid, respectively (Huskins 1946; Sears 1952,1954; Murainatsu 1963). Muraniatsu (1963) showed that four or fewer doses of ssp. spelta (q) chromosome 5A substituted into the Chinese Spring background resulted in a speltoid spike, but five doses of ssp. spelta 5A (qqqqq) gave a squareheaded spike that corresponded approximately to two doses of chromosome 5A from euploid Chinese Spring (QQ). This finding indicated that q was not a deficiency, but an allele with the same effect as Q only to a lesser degree. In this sense, Muramatsu (1963) concluded that q was hypomorphic to Q, and that the threshold for squareheadedness must he somewhere between four q's and five q's.
Q in the tetraploids: Watkins (1928,1940) reported that, with the exception of T. turgidum ssp. carthlicum, all the tetraploid wheats had keeled glumes and therefore possess K, which was later determined to be the same as q (MacKey 1954). Therefore, the tetraploid wheats were considered to possess the q allele even though some were known to be free threshing. Muramatsu (1978, 1979, 1985, 1986) conducted experiments with several tetraploid species to determine if they carried Q or q. By observing the effects of chromosome 5A of the tetraploids in the Chinese Spring background, he showed that not only is ssp. carthlicum of QQ genotype, but also ssp. polonicum, ssp. durum, and ssp. dicoccum var. liguliforme, which is a spelting line with keeled glumes and brittle rachis, but has dense compact spikes. In addition, he demonstrated that ssp. dicoccum var. farrum cvs. Large White emmer and Vernal emmer possess q. Based, on these experiments, Muramatsu (1986) concluded that there is wide phenotypic variation of characters in different QQ lines, and suggested that the range of variation of these characters is very narrow in the absence of Q, but when Q is present they express an obvious phenotype.
Q in the hexaploids: Due to their relatively recent origin, the evolution of the hexaploids is simpler compared to the complexity observed with the tetraploids. Not all hexaploid cultivars that have Q have round glumes. Some have keels that persist down to the base of the glume. Therefore, as with the tetraploids, one cannot conclude whether a particular hexaploid genotype is Q or q based on glume keeledness alone due to potential modification by the genetic background. In addition, the square headed phenotype is not always displayed in QQ genotypes. Sears (1956) attributed the difference between squareheadedness and non-squareheadedness to genes that modify Q rather than any difference in Q itself. He demonstrated this by substituting chromosome 5A from the non-squareheaded variety 'Hope' into the squareheaded variety Chinese Spring. The resulting plant was as squareheaded as Chinese Spring indicating that Chinese Spring provides a background that leads to squareheadedness in the presence of two Q alleles. Muramatsu (1963, 1986) made it clear that the ambiguities of Q -influenced characters between ssp. aestivum and ssp. spelta are due to the pleiotropic nature of Q /q and the modifying effect of the genetic background. The dosage of Q or q required to elevate the phenotype above the aestivum threshold depends on the intensity of genetic modifiers in the background and which of the pleiotropic effects of Q is being considered. For example, in Chinese Spring a relatively low dosage of Q is required to produce squareheadedness, while non- squareheaded aestivum varieties require a higher dosage to reach squareheadedness. In essence, it is possible for a genotype to possess Q but display a speltoid phenotype closely resembling that of q given appropriate factors are present in the genetic background.
Hypotheses regarding the rise of Q : As mentioned above, the primary cultivated wheats, T. turgidum ssp. durum and T. aestivum ssp. aestivum, are free threshing and have the Q Q genotype. Both species are presumed to have evolved through a q -tetraploid such as the primitive wheat T. turgidum ssp. dicoccum, Kuckuck (1959) suggested that ssp. aestivum originated through duplication of q to produce Q because a cross between two ssp. spelta lines resulted in some progeny with an aestivum phenotype. Swaminathan (1963) suggested that the Q locus consisted of tandem repeats and unequal crossing over within the locus could give rise to speltoids and compactoids. It has also been suggested that Q arose from q through mutation and vice versa (Muramatsu 1963), but it is not known whether the same mutations would have occurred independently in Q -tetraploids and T. aestivum ssp. aestivum or if a Q -tetraploid was involved in the origin of hexaploid wheat.
Positional cloning of Q
Physical chromosome mapping: Several studies have reported on the physical mapping of the Q gene to the long arm of chromosome 5A using chromosome deletion lines (Miller and Reader 1982; Endo and Mukai 1988; Tsujimoto and Noda 1989, 1990; Ogihara et al. 1994; Endo and Gill 1996). Others have developed recombination-based linkage maps for chromosome 5A that included the Q locus (Kojima and Ogihara 1998; Kate et al. 1999).
Endo and Gill (1996) physically mapped Q within a cytological submicroscopic deletion flanked by overlapping breakpoints in deletion lines 5AL-7 and 5AL-23. This deletion interval accounted for less than 1% of the physical size of 5AL, and the two deletions could be differentiated only by phenotype, i.e. 5AL-7 had a speltoid spike indicating the absence of Q while 5AL-23 had a square head. Gill et al. (1996) employed the chromosome deletion lines to generate physical maps of the homoeologous group 5 chromosomes, but no molecular markers within the Q gene deletion interval were identified. Therefore, we (Fans and Gill 2001) devised a strategy to target molecular markers to the Q gene deletion interval by employing the flanking deletion lines 5AL-7 and 5AL-23 in combination with molecular techniques such as restriction fragment length polymorphisms (RFLPs) (Botstein et al. 1980), mRNA differential display (Liang and Pardee 1992), and amplified fragment length polymorphisms (AFLPs) (Vos et al. 1995).
Consensus maps incorporating loci from the homoeologous wheat genomes and the corresponding chromosomes of barley (Hordeum vulgare), Ae. tauschii, and T. monococcum (Van Deynze et al. 1995a, b; Nelson et al. 1995a, b, c) have revealed that the genomes of these related species are essentially colinear with the homoeologous wheat genomes. Therefore, we surveyed numerous maps of multiple related species to identify RFLP probes that would potentially detect fragments near the Q locus on wheat 5AL. Of 135 probes surveyed, six detected fragments within the Q gene deletion interval (Fig. 2).
For AFLP analysis, we surveyed over 200 primer combinations and identified 26 fragments that amplified in 5AL-23 but not 5AL-7. These positive fragments were cloned and converted to RFLP probes. While many of the positive fragments represented repetitive sequences, we identified nine that were low-copy and detected fragments within the Q gene deletion interval (Fig. 2).
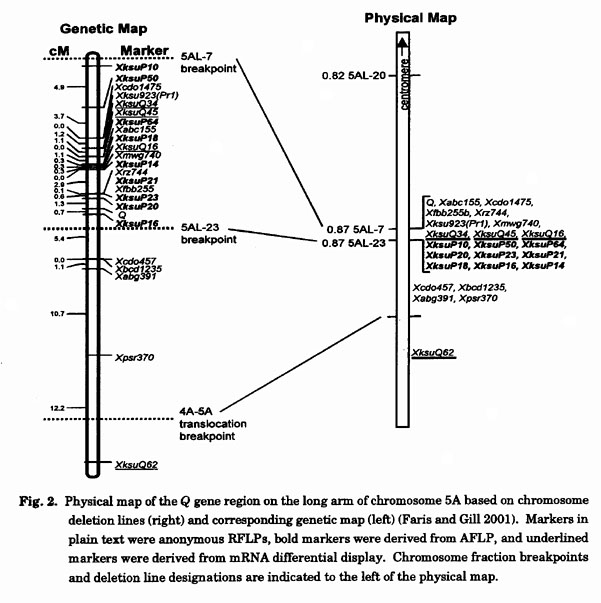
We surveyed 90 primer combinations using mRNA differential display and found 16 bands that were expressed in 5AL-23 but not 5AL-7. Cloning and characterization of these bands resulted in the 92 unique clones, indicating that many differentially expressed fragments comigrated in the polyacrylamide gels. Ultimately, we identified only three differential display-derived probes that mapped within the Q gene deletion interval (Fig. 2). However, many differential expressed genes were present on the homoeologous group 5 chromosomes suggesting they, and others, were likely under the influence of a regulatory gene(s) within the Q gene deletion interval.
Fine-genetic mapping: The combination of RFLP, AFLP, and differential display yielded a total of 18 molecular markers within the Q gene deletion interval. Using these markers, we constructed a genetic linkage map corresponding to the deletion interval in a population of 465 F2 plants derived from either Chinese Spring (CS) x CS-T. turgidum ssp. dicoccoides chromosome 5A disomic substitution (CS-DIC 5A), or from CS-T. aestivum cv. Cheyenne chromosome 5A disomic substitution (CS-CNN 5A) x CS-DIC 5A. The resulting linkage map spanning the distance between the breakpoints in deletion lines 5AL-7 and 5AL-23 accounted for about 20 cM (Fig. 2). The Q gene mapped toward the distal end of the linkage segment and was flanked by AFLP-derived markers XksuP23 and XksuPl6 at distances of 1.3 and 0.7 cM, respectively.
Comparisons with previously published linkage maps of chromosome 5A suggested that the 5AL-7/23 deletion interval accounted for about 8 to 15% of the recombination that occurs on chromosome 5A. We estimated that the average recombination frequency within the interval is approximately 250 kb/cM, which is an 18-fold increase compared to the genomic average but agreed with other reports indicating that gene-rich regions tend to be hot spots for recombination (Feuillet and Keller 1999; Spielmeyer et al. 2000; Stein et al. 2000; Brooks et al. 2002).
Chromosome walking at the Q locus: Chromosome walking in wheat had long been considered infeasible due to the size of the wheat genome and the large amount of repetitive DNA. But the plethora of data indicating that most of the recombination occurs within gene-rich islands along the wheat chromosomes provided new hope for positional cloning in wheat. Stein et al. (2000) were the first to demonstrate that chromosome walking was feasible. They employed a subgenome walking approach using a T. monococcum BAC library (Lijavetsky et al. 1999) and constructed a 450 kb contig spanning the Lr10 locus in hexaploid wheat. Since then, a number of labs have reported success in chromosome walking (Fans et al. 2003; Yan et al. 2003, 2004; Distelfeld et al. 2004; Yahiaoui et al. 2004) and even chromosome landing (Huang et al. 2003) in wheat.
For physical mapping of the Q locus (Fans et al. 2003), we used the T. monococcum BAC library taking an approach similar to that of Stein et al. (2000) except that we completely sequenced BACs instead of conducting low-pass sequencing. We initiated the walk by screening the library with XksuPl6, which was the marker most closely linked to Q based on the results of fine-mapping (Fig. 2). One positive BAC was identified, completely sequenced, and surveyed for putative low-copy segments that could be used as probes to rescreen the library and extend the contig. Using this approach, we performed a total of three chromosome walking steps resulting in a contig of four BACs that spanned a physical distance of about 300 kb, which corresponded to a genetic distance of 0.9 cM (Fig. 3). Thus, the average physical to genetic ratio within the contig was about 330 kb/cM. However, we could not verify that our contig spanned the Q locus based on recombinants alone, because the proximal 80 kb of the contig, which included three markers, cosegregated with the Q gene.
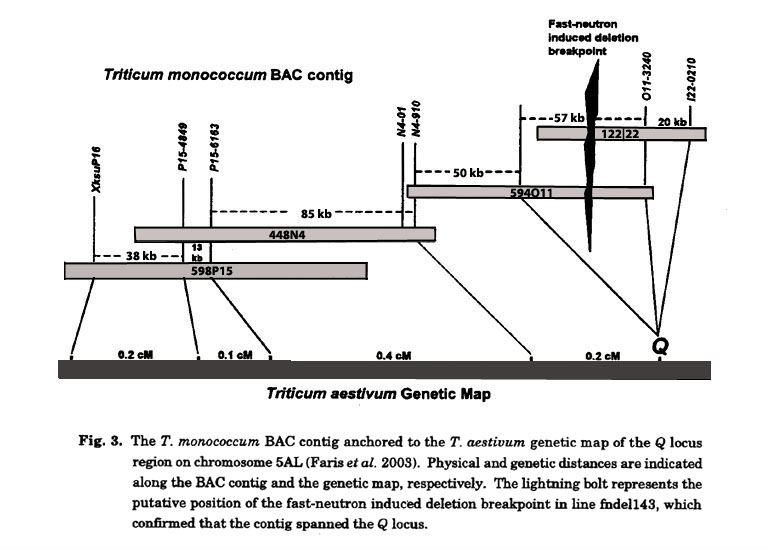
Analysis of fast-neutron induced speltoid mutants: At the same time that chromosome walking was being performed, we analyzed several thousand CS mutants treated with fast neutrons. We identified 26 putative speltoid mutants and assessed them for deletions at the Q locus using markers derived from the BAC contig. Most of the speltoid mutants had large chromosome deletions, and some were missing much of the long arm of chromosome 5A. The smallest deletion was observed in plant fndel-143, which displayed a classic speltoid phenotype indicating the Q gene was absent. Molecular analysis of fndel-143 indicated that marker 011-Tmap2, which cosegregated with Q, and the rest of the distal markers in the BAC contig were absent from this mutant. On the contrary, the proximal markers including 011-3240, which also cosegregated with Q, were present. Therefore, the proximal deletion breakpoint in fndel-143 physically separated 011-3240 from Q and eliminated anything proximal to 011-3240 as a candidate for the Q gene. This result confirmed that our T. monococcum BAC contig spanned the Q locus and narrowed the region for prospective Q gene candidates to a segment between N4-910 and 011-3240, which was a segment of approximately 100 kb (Fig. 3).
Cloning and validation of the Q gene: Within the 100 kb candidate region, there was a predicted 3.5 kb open reading frame (ORF) with a high degree of similarity to the maize indeterminant spikelet (ids) gene (Chuck et al. 1998), APETALA2 (AP2) in Arabidopsis (Jofuku et al. 1994), and other AP2-like genes. AP2 and AP2-like genes are floral homeotic transcription factors distinguished by plant-specific DNA binding motifs referred to as AP2 domains, and these genes have been implicated in a wide range of plant development roles including the establishment of floral meristem identity (Irish and Sussex 1990; Bowman et al. 1993), the specification of floral organ identity (Komaki et al. 1988; Bowman et al. 1989; Kunst et al. 1989; Jofuku et al. 1994), and the temporal and spatial regulation of floral homeotic gene expression (Drews et al. 1991). These functional properties of characterized AP2-like genes suggested that a wheat AP2 homolog was a likely candidate for Q . However, because T. monoccum is non free threshing and has spelting properties it is considered to possess the q allele. Therefore, we obtained the genomic sequence of the wheat AP2-like (WAP2) gene from wild type CS and the ssp. aestivum cv. Bobwhite (BW) using PCR, and determined the full-length coding region using a combination of reverse transcription (RT)-PCR, 5'-and 3'-RACE, and searches against the wheat EST databases (Fig. 4). The WAP2 gene consists of 10 exons and 9 introns, extends 3,229 bp from start to stop codon, and has a GC content of 54%. A plant AP2-like promoter is 46 bp upstream of the start codon. The coding sequence is 1,344 bp long plus a 138 bp 5' UTR and a 255 bp 3' UTR. It encodes a protein of 447 amino acids.
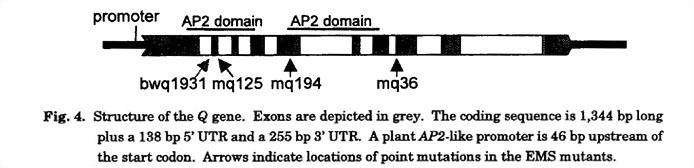
To validate that the WAP2 gene was Q , we generated populations of EMS mutants in the CS and BW backgrounds and screened them for speltoid phenotypes. Three speltoid mutants were recovered in the CS population, and sequencing and subsequent RT-PCR analysis revealed that one mutant had an altered amino acid within an PA2 domain, and the other two had base changes that led to alternate splicing during transcription. One speltoid mutant was recovered in the BW population, which harbored a point mutation in the first nucleotide of exon 2 leading to an altered amino acid in an AP2 domain. Thus, this analysis confirmed that the WAP2 candidate gene was Q .
Structural and functional analysis of Q
Comparisons of Q and q: The isolation of Q allowed us to address some of the historical hypotheses formulated by researchers some decades ago. Regarding the origin of Q , some suggested that it arose as a duplication of q (Kuckuck 1959; Swanunathan 1963), while others speculated that Q arose from q by mutation (Muramatsu 1963). While there was a valid basis for the formulation of each of these hypotheses, we disproved the former by hybridizing a fragment of the Q gene with multiple non-free-threshing and free-threshing hexaploid and tetraploid genotypes (Fig. 5). Restriction patterns and fragment hybridization intensities suggested that a single copy of the gene is present on each of the homoeologous group 5 chromosomes. In addition, sequence analysis of the 142 kb BAC harboring the Q allele from T. turgidum ssp. durum indicated a single ORF corresponding in size and structure to the q ORF from T. monococcum.

Sequence analysis and the fact that only domesticated species possess Q while the wild relatives have q indicate that Q arose from q through mutation. We examined the genomic sequences of Q alleles from 15 genotypes representing 12 Triticum (sub)species (five with Q and seven with q) and identified six conserved differences that differentiated between Q- and q-genotypes (Fig. 6). One of the nucleotide differences changed a predicted amino acid suggesting that the phenotypic differences between Q and q genotypes might be structural. However, we also used relative quantitative (RQ)-PCR to compare the level of expression between Q in CS and q in CS-DIC 5A in various stages of spike development. Gene expression in both CS and CS-DIC 5A peaked in the early stages of spike development and gradually declined as the spikes matured (Fig. 7a). While expression patterns for both Q and q were quite similar, the expression level of Q was consistently higher than q. Therefore, the mechanism(s) underlying the difference in functional regulation and the mutation that gave rise to Q have yet to be identified.

Both Q and q are expressed in leaves and roots, but as with spikes, q transcripts are less abundant compared to Q (Fig. 7b). Expression of Q in leaves is the same as in spikes, while q is apparently expressed to higher levels in leaves than in spikes. Expression levels of both Q and q are much less in roots compared to leaves and spikes, but the abundance of Q is about 2-fold compared to that of q.

Dosage and pleiotropic effects of Q: The early work describing dosage and pleiotropic effects of Q was conducted using whole chromosome substitution lines. The cloning of Q allowed us to investigate effects of the gene itself through ectopic expression. We transformed the T. aestivum ssp. aestivum cv. BW, which has two endogenous copies of Q, with the T. turgidum ssp. durum cv. Langdon (LDN) Q allele driven by its native promoter. Transgenic plants exhibited various spike morphologies ranging from severely speltoid to extremely compactoid (Fig. 8). The speltoid transgenics harbored a large number of transgene copies, which led to post-transcriptional gene silencing of Q (Fig. 9). On the contrary, compactoid transgenics had fewer integration events but a much higher level of Q gene expression. These results confirmed the dosage effects of Q on spike density reported by researchers a half century ago (Huskins 1946; Sears 1952, 1954; Muramatsu 1963).
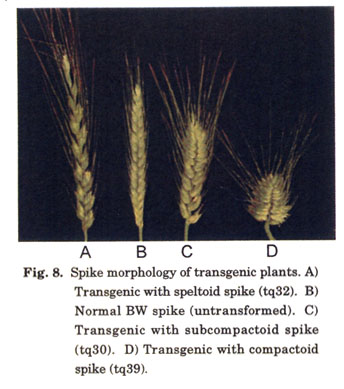
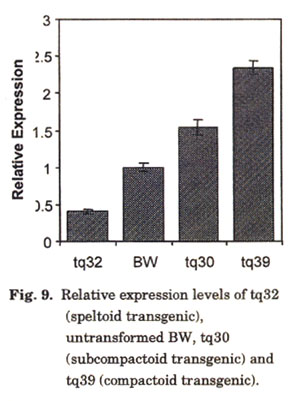
In addition to spike density, other traits were also correlated with the level of Q gene expression. Plants with silenced Q not only had speltoid spikes, but were also taller, had keeled tenacious glumes that adhered strongly to the seed, and fragile rachises, in which the disarticulation pattern was such that the rachis broke above the junction of the rachis and rachilla leaving a portion of the rachis at the base of the spikelet. Expression of multiple copies of Q led to increasingly shorter plants. All Q -expressing plants had tough rachises and round, soft glumes that loosely held the seed, and were free-threshing.
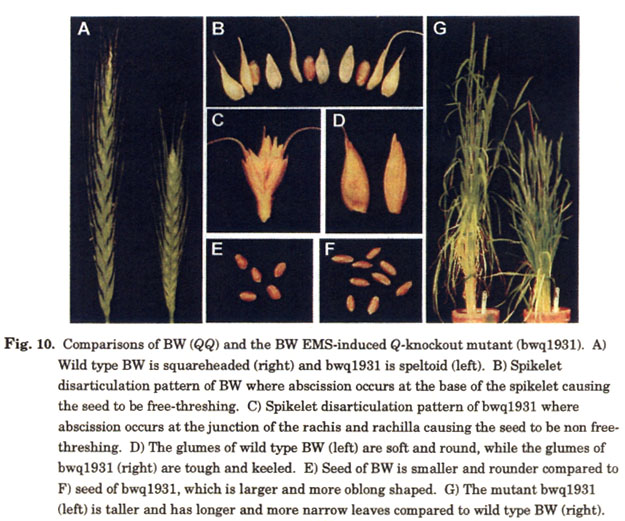
Phenotypic comparisons of the knockout mutant in BW with the wild type also revealed further functions of Q . In addition to having an elongated speltoid spike with nonfree-threshing seed and tough glumes (Fig. 10-a,b,c,d), the knockout mutant was taller, had longer, thinner leaves (Fig. 10g), flowered earlier, and produced slightly larger seed compared to the wild type (Fig. 10e,f). It is interesting to note that AP2-knockouts in Arabidopsis have also been shown to have larger seed size (Ohto et al. 2005; Jofuku et al. 2005).
Micro-colinearity of the Q region with rice
Much effort has been put forth in comparing the genomic relationships among grasses (Devos and Gale 2000). Comparative mapping experiments among wheat and other members of the Poaceae like rice, barley, rye, oats, and maize have revealed remarkable similarities in gene content and marker synteny at the chromosome level. However, studies of the degree of micro-colinearity between rice and wheat have showed less promise indicating that it maybe difficult to use rice genomics for gene discovery in wheat (Sorrells et al. 2003).
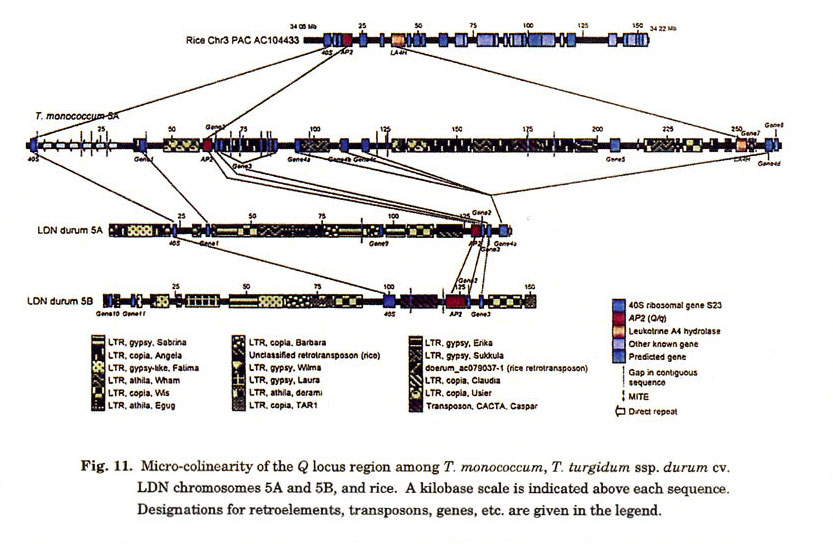
The 300 kb T. monococcum BAC contig spanning the Q locus allowed us to make direct comparisons with sequences in the rice genome (Fig. 11). Annotation of the contig revealed three ORFs with similarity to known genes in Genbank and included the AP2-like gene (Q ), a leukotrine A4 hydrolase (LA4H), and a 40S ribosomal gene (40S). Homologous sequences of all three genes were identified in close proximity of each other on a single rice PAC (AC104433), while they were separated by much larger distances in T. monococcum due to interspersion of retroelements, direct repeats, and several predicted ORFs. Whether or not the predicted ORFs represent functional genes is unknown, but one putative gene (Gene4) was present in four copies on the T. monococcum contig. None of the predicted genes detected homologous sequences in the rice genome, and similarly, predicted genes interspersed among the 40S, Q , and LA4H homologs in rice were not present on the T. monococcum BAC contig.
To extend the comparative analysis, we sequenced homoeologous chromosome 5A and 5B BACs spanning the Q homeoalleles from T. turgidum ssp. durum cv. LDN (Cenci et al. 2003) (Fig. 11). While neither BAC extended so far as to contain the LA4H gene, both possessed homologs of 40S and Q . In addition, homologous sequences of predicted genes Gene1, Gene2, Gene3, and Gene4 found in T. monococcum were present in the LND 5A BAC, while homoeologs of only Gene2 and Gene3 were present in the LDN 5B BAC.
It is also interesting to note the distances and intervening elements that separate 40S and Q homologs among the three sequences (Fig. 11). The two genes are separated by distances of 60 kb in T. monococcum, 110 kb in LDN 5A and 20 kb in LDN 5B. Two retrotransposons and a large number of direct repeats account for most of the distance between 40S and the q allele in T. monococcum. Seven different types of retrotransposons have inflated this intergenic region in LDN 5A, while only one transposon separates the two genes in LDN 5B. This suggests that while gene order and gene content may or may not be conserved among homoeologous genomes, the intergenic distances and sequences are highly variable.
Q and the origin of cultivated wheat
It is well known that the A-genome donor of tetraploid and hexaploid wheats is T. urartu (Dvorak et al. 1993), and the D-genome donor of hexaploid wheat is Ae. tauschii (Kihara 1944; McFadden and Sears 1946). However, the AB tetraploid involved in the hybridization with Ae. tauschii (D genome) that gave rise to hexaploid wheat has been a matter of controversy. And, with regards to Q, it has been a matter of speculation whether it first arose in the tetraploid progenitor of hexaploid wheat, or if it arose independently in hexaploids and tetraploids.
The genes Br (BRITTLE RACHIS) and Tg control rachis fragility and glume toughness, respectively. While the Q gene governs the free-threshing character, it also pleiotropically influences both rachis fragility and glume toughness. Domesticated emmer wheats were presumably homozygous brbrtgtgqq based on hulledness and pattern of disarticulation. Our preliminary phylogenetic analysis (Fig. 12) and sequence analysis based on full-length genomic sequences of Q alleles from multiple (sub)species suggests that Q arose only once. Therefore, a mutation in the q allele likely gave rise to a free threshing tetraploid with genotype brbrtgtgQQ, and this genotype was possibly the tetraploid progenitor of hexaploid wheat.
Among the spelt wheats, Luo et al. (2000) showed that Iranian T. aestivum ssp. spelta possesses Q while European spelta possesses q. This agrees with our sequence and phylogenetic results (Fig. 12). Iranian spelta falls in the clade with the other Q-genotypes while European and Swiss spelta (q) are more distant and clade closer with the q-containing tetraploids T. turgidum spp. dicoccum and dicoccoides (Fig. 12). Others have presented strong evidence that European spelta resulted from hybridization between a free-threshing hexaploid with Q and a tetraploid with q such as T. turgidum ssp. dicoccum, thus giving rise to a hexaploid with q (Liu and Tsunewaki 1991; Blatter et al . 2004). Yan Y et al . (2003) suggested that the putative hexaploid progenitor of European spelta was T. aestivum ssp. compactum .

The archaeological record indicates remnants of free-threshing tetraploid and hexaploid wheats appear about a thousand years earlier than spelt wheats suggesting that neither Iranian spelta or European spelta are progenitors of free-threshing hexaploid wheat (reviewed in Feldman 2001). One scenario for the origin of hexaploid wheat would presume that a Q -tetraploid similar to present day T. turgidum spp. or the extinct tetraploid naked wheat T. turgidum ssp. parvicoccum probably hybridized with Ae. tauschii in some farmer's field. At that time, a mixture of cytotypes of einkorn, emmer, and probably spp. parvicoccum was grown and a TgTg Q Q hexaploid became a part of the wheat crop. Therefore, the first hexaploid wheat was similar to the T. aestivum ssp. spelta and probably experienced a mutation of the Ae. tauschii -derived Tg to tg soon after the amphiploidization event. The resulting free-threshing hexaploid was selected and spread readily because of its acquired domestication trait. Under this scenario, the present day spelt wheats originated later through hybridization of free-threshing T. aestivum ssp. aestivum with tetraploid wheats, while the original hexaploid has not been discovered or has become extinct. Some Q - containing tetraploids may have also been secondarily derived from such hybridization events, particularly ssp. carthlicum which is presumed to have originated relatively late due to its narrow distribution.
Alternatively, domesticated emmer wheat may be the tetraploid progenitor of bread wheat. Under this scenario, emmer wheat hybridized with Ae. tauschii giving rise to a TgTgqq hexaploid, which would have quickly undergone mutations at both loci to produce a free-threshing tgtg Q Q hexaploid. While this scenario would seem unlikely, it has been shown that rapid and significant changes occur at the early stages of allopolyploidization (Feldman et al . 1997). Its also possible that Q arose first in emmer wheat based on the existence of the Q -bearing T. turgidum ssp. dicoccum var. liguliforme (Muramatsu 1979). However, it is conceivable that var. liguliforme obtained Q through hybridization with a Q -hexaploid.
Future prospects for Q
The regulatory nature of the Q gene was realized decades ago, and now that we have isolated Q, we can begin to decipher the mechanisms, genetic interactions, and biochemical pathways associated with Q and its pleiotropic effects. In Arabidopsis, AP2 has been extensively studied for the past decade. An interesting aspect of AP2 in Arabidopsis is that it works at several levels of the gene hierarchy that regulates flower development (Theissen and Saedler 1999, for review). Not only does it function in determining floral meristem identity, it also is a negative regulator of the floral homeotic gene AGAMOUS (AG) in the floral perianth. AP2 is also involved in the specification of sepal and petal identity, the formation of ovules and seeds (Drews et al. 1991), and has recently been implicated in determining seed size and yield (Ohto et al. 2005; Jofuku et al. 2005). It is likely that Q functions in a similar manner in wheat, and further studies will shed light on this notion. However, a more interesting question for wheat biologists may pertain to how Q functions differently from q. As q is the more primitive allele, it likely shares at least some of the same functional properties as its AP2 homologs. But with regards to wheat domestication, it is the difference in function between Q and q and the underlying mechanism(s) that are of greatest interest.
The sequencing of Q alleles from additional Triticum (sub)species and accessions will provide additional knowledge regarding the role of structural differences within the coding region and regulatory elements. Further transformation studies may also be useful to investigate the role of structural differences, however such experiments will likely be confounded by the dosage effects of both Q and q. Additional expression studies will be useful to determine if Q and q are differentially expressed in various tissues and genotypes. The use of cytogenetic stocks that vary for the copy number of chromosome 5A carrying Q or q will be most useful in determining if the difference in expression level between Q and q is responsible for the phenotypic variation. Modern molecular tools such as the yeast two-hybrid system and DNA microarray technology will help us identify genes that directly interact with Q as well as those whose expression is up- or down-regulated by Q. The isolation and characterization of genes such as Tg, Br, CLUB (C), and SPHAEROCOCCUM (S) will lead to a description of the network and pathways that are responsible for spike morphology and domestication traits.
From a practical standpoint, the source of sustenance derived from wheat is obtained from the grain. Therefore, understanding the genetic basis of spike development may further our ability to transform inflorescences and flowers for crop (and food) improvement. Because Q influences a repertoire of domestication-related traits, it is conceivable that it could be used for crop development. When placed with the appropriate gene ensemble, Q may be exploited to domesticate wild species for specific valuable traits and end-use products. Regardless of what the future holds for Q, it has already solidified its place in history as one of the most important agricultural genes in the rise of modern civilization and as a testament of human ingenuity.
Acknowledgements
We acknowledge and thank Dr. John Fellers for providing DNA sequencing services and Dr. Harold Trick for performing transformation. We thank E. Doehler, D. Wilson, J. Kuehn, V. Kuraparthy, A. Mathews, M. Herbel, K Gleason, J. Essig, M. Main, and M. Schapaugh for technical assistance. This research was supported by USDA-ARS CRIS 5442-21000-030-OOD and special USDA grant to the Wheat Genetics Resource Center at Kansas State University (to B.S.G.).
References
Blatter RHE, Jacomet S and Schlumbaum A (2004) About the origin of European spelt (Triticum spelta L.): allelic differentiation of the HMW glutenin B1-1 and A1-2 subunit genes. Theor Appl Genet 108:360-367.
Botstein D, White RL, Skolnick M and Davis RW (1980) Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am J Hum Genet 32: 314-331.
Bowman JL, Smyth DR and Meyerowitz EM (1989) Genes directing flower development in Arabidopsis. Plant Cell 1: 37-52.
Bowman JL, Alvarez J, Weigel D, Meyerowitz EM and Smyth DR (1993) Control of flower development in Arabidopsis thaliana by APETALA1 and interacting genes. Development 119: 721-743.
Brooks SA, Huang L, Gill BS and Fellers JP (2002) Analysis of 106 kb of contiguous DNA sequence from the D genome of wheat reveals high gene density and a complex arrangement of genes related to disease resistance. Genome 45: 963-972.
Cenci A, Chantret N, Kong X, Gu Y, Anderson OD, Fahima T, Distelfeld A and Dubcovsky J (2003) Construction and characterization of a half million clone BAC library of durum wheat (Triticum turgidum ssp. durum). Theor Appl Genet 107:931-939.
Chuck G, Meeley RB and Hake S (1998) The control of maize spikelet meristem fate by the APETALA2- like gene indeterminate spikelet1. Genes Devel 12: 1145-1154.
Devos KM and Gale MD (2000) Genome relationships: The grass model in current research. Plant Cell 12: 637-646.
Distelfeld A, Uauy C, Olmos S, Schlatter AR, Dubcovsky J and Fahima T (2004) Microcolinearity between a 2-cM region encompassing the grain protein content locus Gpc.6B1 on wheat chromosome 6B and a 350-kb region on rice chromosome 2. Funct Integr Genomics 4: 59-66.
Drews GN, Bowman JL and Meyerowitz EM (1991) Negative regulation of the Arabidopsis homeotic gene AGAMOUS by the APE TALA2 product. Cell 65:991-1002.
Dvorak J, di Terlizzi P, Zhang HB and Resta P (1993) The evolution of polyploid wheats: identification of the A genome donor species. Genome 36: 21-31.
Endo TR and Gill BS (1996) The deletion stocks of common wheat. J Hered 87: 295-307.
Endo TR and Mukai Y (1988) Chromosome mapping of speltoid suppression gene of Triticum aestivum L. based on partial deletion in the long arm of chromosome 5A. Jpn J Genet 63: 501-506.
Faris JD and Gill BS (2001) Genomic targeting and high-resolution mapping of the domestication gene Q in wheat. Genome 45: 706-718.
Faris JD, Fellers JP, Brooks SA and Gill BS (2003) A bacterial artificial chromosome contig spanning the major domestication locus Q in wheat and identification of a candidate gene. Genetics 164: 311-321.
Feldman MF (2001) Origin of cultivated wheat. In: Bonjean AP and Angus WJ (eds.) The World Wheat Book, A history of wheat breeding. Lavoisier Publishing, Paris, France, pp. 1-56.
Feldman M, Liu B, Segal G, Abbo S, Levy A and Vega JM (1997) Rapid elimination of low-copy DNA sequences in polyploid wheat: a possible mechanism for differentiation of homoeologous chromosomes. Genetics 147: 1381-1387.
Feuillet C and Keller B (1999) High gene density is conserved at syntenic loci of small and large grass genomes. Proc Natl Acad Sci USA 96: 8265-8270.
Gill KS, Gill BS, Endo TR and Boiko EV (1996) Identification and high-density mapping of gene-rich regions in chromosome group 5 of wheat. Genetics 143: 1001-1012.
Heun M, Schaefer-Pregl R, Klawan D, Castagna R, Accerbi M, Borghi B and Salamini F (1997) Site of einkorn wheat domestication identified by DNA fingerprinting. Science 278: 1312-1314.
Huang L, Brooks SA, Li W, Fellers JP, Trick HN and Gill BS (2003) Map-based cloning of leaf rust resistance gene Lr21 from the large and polyploid genome of bread wheat. Genetics 164: 655-664.
Huang S, Sirikhachornkit A, Su X, Fans J, Gill B, Haselkorn R and Gornicki P (2002) Genes encoding plastid acetyl-CoA carboxylase and 3-phosphoglycerate kinase of the Triticum/Aegilops complex and the evolutionary history of polyploid wheat. Proc Natl Acad Sci USA 99:8133-8138.
Huskins CL (1946) Fatuoid, speltoid and related mutations of oats and wheat. Botan Rev 12: 457- 514.
Irish VF and Sussex IM (1990) Function of the apetala-1 gene during Arabidopsis floral development. Plant Cell 2: 741-753.
Jantasuriyarat C, Vales MI, Watson CJW and Riera-Lizarazu 0(2004) Identification and mapping of genetic loci affecting the free-threshing habit and spike compactness in wheat (Triticum aestivum L.). Theor AppI Genet 108: 261-273.
Jofuku KD, den Boer BGW, Van Montagu M and Okamuro JK (1994) Control of Arabidopsis flower and seed development by the homeotic gene APETALA2. Plant Cell 6: 1211-1225.
Jofuku KD, Omidyar PK, Gee Z and Okamuro JK (2005) Control of seed mass and seed yield by the floral homeotic gene APETALA2. Proc Natl Acad Sci USA 102: 3117-3122.
Kato K, Miura H and Sawada S (1999) QTL mapping of genes controlling ear emergence time and plant height on chromosome 5A of wheat. Theor Appl Genet 98: 472-476 .
Kato K, Sonokawa R, Miura H and Sawada S (2003) Dwarfing effect associated with the threshability gene Q on wheat chromosome 5A. Plant Breed 122: 489-492.
Kerber RE and Rowland GG (1974) Origin of the threshing character in hexaploid wheat. Can. J. Genet. Cytol. 16: 145-154.
Kihara H (1944) Discovery of the DD-analyser, one of the ancestors of Triticum vulgare. Agric Hortic 19:13-14.
Kojima T and Ogihara Y (1998) High-resolution RFLP map of the long arm of chromosome 5A in wheats and its synteny among cereals. Genes Genet Syst 73:51-58.
Komaki MK, Okada K, Nishino E and Shimura Y (1988) Isolation and characterization of novel mutants of Arabidopsis thaliana defective in flower development. Development 104: 195-203.
Kuckuck H (1959) Neuere Arbeiten zur Entstehung der hexaploiden Kulturweizen. Z. Pflanzenzucht 41: 205-226.
Kunst L, Klenz JE, Martinez-Zapater J and Haughn GW (1989)AP2 gene detemines the identity of perianth organs in flowers of Arabidopsis thaliana. Plant Cell 1: 1195-1208.
Leighty CE and Boshnakian S (1921) Genetic behaviour of the spelt form in crosses between Triticum spelta and Triticum aestivum. J Agric Res 7: 335-364.
Liang P and Pardee AB (1992) Differential display of eukaryotic messenger RNA by means of polymerase chain reaction. Science 257: 967-971.
Lijavetsky D, Muzzi G, Wicker T, Keller B, Wing R and Dubcovsky J (1999) Construction and characterization of a bacterial artificial chromosome (BAC) library for the A genome of wheat.Genome 42: 1176-1182.
Liu YG and Tsunewaki K (1991) Restriction fragment length polymorphism (RFLP) analysis in wheat. II. Linkage analysis of the RFLP sites in common wheat. Jpn J Genet 66: 617-633.
Luo MC, Yang ZL and Dvorak J (2000) The Q locus of Iranian and European spelt wheat. Theor Appl Genet 100: 602-606.
MacKey J (1954) Neutron and X-ray experiments in wheat and revision of the speltoid problem. Hereditas 40: 65-180.
MacKey J (1966) Species relationship in Triticum. Proc 2nd Intl Wheat Genet Symp Lund Hereditas (suppl): 237-376.
McFadden ES and Sears ER (1946) The origin of Triticum spelta and its free-threshing hexaploid relatives. J Hered 37: 81-89.
Miller TE and Reader SM (1982) A major deletion of part or chromosome 5A of Triticum aestivum. Wheat Inf Serv 88: 10-12.
Muramatsu M (1963) Dosage effect of the spelta gene q of hexaploid wheat. Genetics 48: 469-482.
Muramatsu M (1978) Phenotypic expression of the vulgare gene Q in tetraploid wheat. Proc V Int Wheat Genet Symp, New Delhi 1: 92-102.
Muramatsu M (1979) Presence of the vulgare gene, Q, in a dense-spike variety of Triticum dicoccum Schubl. Report of the Plant Germ-Plasm Institute, Kyoto University, No. 4. pp. 39-41.
Muramatsu M (1985) Spike type in two cultivars of Triticum dicoccum with the spelta gene q compared with the Q-bearing variety liguliforme. Jpn J Breed 35: 255-267.
Muramatsu M (1986) The vulgare super gene, Q: its universality in durum wheat and its phenotypic effects in tetraploid and hexaploid wheats. Can J Genet Cytol 28: 30-41.
Nelson JC, Sorrells ME, Van Deynze AE, Lu YH, Atkinson M, Bernard M, Leroy P, Fans JD and Anderson JA (1995a) Molecular mapping of wheat. Major genes and rearrangements in homoeologous groups 4, 5, and 7. Genetics 141:721-731.
Nelson JC, Van Deynze AE, Autrique E, Sorrells ME, Lu YH, Merlino M, Atkinson M and Leroy P (1995b) Molecular mapping of wheat. Homoeologous group 2. Genome 38: 516-524.
Nelson JC, Van Deynze AE, Autrique E, Sorrells ME, Lu YH, Negre S, Bernard M and Leroy P (1995c) Molecular mapping of wheat. Homoeologous group 3. Genome 38: 525-533.
Nilsson-Ehle H (1917) Untersuchungen uber speltoid mutationen beim weizen. Botan Notiser 305: 3020
Nilsson-Ehle H (1920) Multiple allelomorphe und complex mutationen beim weizen. Hereditas 1: 277-311
Ogihara Y, Hasegawa K and Tsujmioto H (1994) High-resolution cytological mapping of the long arm of chromosome 5A in common wheat using a series of deletion lines induced by gametocidal (Gc) genes of Aegilops speltoides. Mol Gen Genet 244: 253-259.
Ohto MA, Fischer RL, Goldberg RB, Nakamura K and Harada JJ (2005) Control of seed mass by APETALA2. Proc Natl Acad Sci USA 102: 3123-3128.
Ozkan H, Brandolini A, Schafer-Pregl R and Salamini F (2002) AFLP analysis of a collection of teraploid wheatons indicates the origin of emmer and hard wheat domestication in Southeast Turkey. Mol Biol Evol 19: 1707-1801.
Sears ER (1952) Misdivision of univalents in common wheat. Chromosoma 4:535-550.
Sears ER (1954) The aneuploids of common wheat. Missouri Agr Exp Sta Res Bull 572: 59 pp.
Sears ER (1956) The systematics, cytology and genetics of wheat. Handb Pflanzenzucht, 2 Edition, 2:164-187.
Sears ER (1958) The aneuploids of common wheat. Proc 1st Intl Wheat Genet Symp Winnipeg, CA pp. 221-229.
Singh MP (1969) Some radiation induced changes at 'Q' locus in bread wheat (Triticum aestivum L.) Caryologia 22: 119-126.
Singh HB, Anderson E and Pal BP (1957) Studies in the genetics of Triticum vavilovii Jackub. Agron J 49: 4-11.
Smith SG, Huskins CL and Sander GF (1949) Mutations in polyploid cereals. II. The cytogenetics of speltoid wheats. Can J Res Com 27: 348-393.
Sorrells ME, La Rote M, Bermudez-Kandianis CE, Greene RA, Kantety R, Munkvold JD, Miftahudin, Mahmoud A, Ma. IC, Gustafson PJ, Qi LL, Echalier B, Gill BS, Matthews DE, Lazo GR, Chao S, Anderson OD, Edwards H, Linkiewicz AM, Dubcovsky J, Akhunov ED, Dvorak J, Zhang D, Nguyen HT, Peng J, Lapitan NL, Gonzalez-Hernandez JL, Anderson JA, Hossain K Kalavacharla V, Kianian SF, Choi DW, Close TJ, Dilbirligi M, Gill KS, Steber C, Walker-Simmons MK McGuire PE and Qualset CO (2003) Comparative DNA sequence analysis of wheat and rice genomes. Genome Res 13: 1818-1827.
Spielmeyer W, Moullet 0, Laroche A and Lagudah E (2000) Highly recombinogemc regions at seed storage protein loci on chromosome IDS of Aegilops tauschii, the D-genome donor of wheat. Genetics 155: 361-367.
Stein N, Feuillet C, Wicker T, Schlagenhauf E and Keller B (2000) Subgenome chromosome walking in wheat: A 450-kb physical contig in Triticum monococcum L. spans the Lr10 resistance locus in hexaploid wheat (Triticum aestivum L.) Proc Natl Acad Sci USA 97: 13436-13441.
Swaminathan MS (1963) Induonced mutations at the Q locus in relation to the phylogeny of hexaploid Triticum species. Proc XI Int Cong Genet The Hague.
Theissen G and Saedler H (1999) The golden decade of molecular floral development (1990-1999): A cheerful obituary. Dev Genet 25: 181-193.
Tsujimoto H and Noda K (1989) Structure of chromosome 5A of wheat speltoid mutants induced by the gametocidal genes of Aegilops speltoides. Genome 32: 1085-1090.
Tsujimoto H and Noda K (1990) Deletion mapping by gametocidal genes in common wheat: position of speltoid suppression (Q) and β-amylase (β-Amy-A2) genes on chromosome 5A. Genome 33: 850-853.
Tsunewaki K and Jenkins BC (1961) Monosomic and conventional analyses of genes in common wheat. II. Jpn J Genet 36:428-443.
Unrau J, Smith WE and McGinnis RC (1950) Spike density, speltoidy and compactoidy in hexaploid wheat. Can J Res Com 28: 273-276.
Van Deynze AE, Dubcovsky J, Gill KS, Nelson JC, Sorrells ME, Dvorak J, Gill BS, Lagudah ES, McCouch SR and Appels R (1995a) Molecular-genetic maps for group 1 chromosomes of Triticeae species and their relation to chromosomes in rice and oat. Genome 38: 45-59.
Van Deynze AE, Nelson JC, Yglesias ES, Harrington SE, Braga DP, McCouch SR and Sorrells ME (1995b) Comparative mapping in grasses. Wheat relationships. Mol Gen Genet 248: 744-754.
Vos P, Hogers R, Bleeker M, Reijans M, Van de Lee T, Homes M, Frijters A, Pot J, Peleman J, Kuiper M and Zabeau M (1995) AFLP: A new technique for DNA fingerprinting. Nucleic Acids Res 23: 4407-4414.
Watkins AE (1927) Genetic and cytological studies in wheat. III. J Genet 18: 375-396.
Watkins AE (1928) The genetics of wheat species crosses. I. J Genet 20: 1-27.
Watkins AE (1930) The wheat species: a critique. J Genet 23: 173-263.
Watkins AE (1940) The inheritance of glume shape in Triticum. J Genet 39: 249-264.
Winge 0(1929) Zytologische untersuchengen uber speltoids und andere mutantenabnliche aberranten beim weizen. Hereditas 5: 241-286
Yahiaoui N, Srichumpa P, Dudler R and Keller B (2004) Genome analysis at different ploidy levels allows cloning of the powdery mildew resistance gene Pm3b from hexaploid wheat. Plant J 37: 528-538.
Yan Y, Hsam SLY., Yu JZ, Jiang Y, Ohtsuka I and Zeller FJ (2003) HMW and LMW glutenin alleles among putative tetraploid and hexaploid European spelt wheat (Triticum spelta L.) progenitors. Theor Appl Genet 107: 1321-1330.
Yan L, Loukoianov A, Tranquilli G, Helguera M, Fahima T and Dubcovsky J (2003) Positional cloning of the wheat vernalization gene VRN1. Proc Natl Acad Sci USA 100: 6263-6268.
Yan L, LoukoianovA, Blechl A, Tranquilli G, Ramakrishna W, SanMiguel P, Bennetzen JL, Echenique V and Dubcovsky J (2004) The wheat VRN2 gene is a flowering repressor down-regulated by vernalization. Science 303: 1640-1644.