Memorial Issue, Wheat Information Service No. 100
Pathways that promote the floral transition in wheat
K. Murai*, C. Ikari and N. Shitsukawa
Department of Bioscience, Fukui Prefectural University, 4-1-1 Kenjojima, Matsuoka-cho,Yoshida-gun, Fukui 910-1195, Japan
* Corresponding author: Koji Murai
E-mail address: murai@fpu.ac.jp
Key words: floral transition, heading time, MADS box gene, WAP1 (Wheat APETALA1), WFT (Wheat FLOWERING LOCUS T), WSOC1 (Wheat SUPPRESSOR OF OVEREXPRESSION OF CO1), WFL (Wheat FLORICAULA /LEAFY)
Introduction
Floral transition, the phase transition from vegetative to reproductive development, is a critical event in the life cycle of seed-propagated plants. In cereal crops, heading time associated with the timing of floral transition is an important character because of its influence on the adaptability to various environmental conditions. Bread wheat (Triticum aestivum L., 2n=6x=42, genome constitution AABBDD) is grown in a wide range of environments all over the world. Its wide adaptability results from the varietal variation in the heading time that is determined by a combination of genotype and environmental stimuli such as day length and temperature. Many genetic studies have been done to clarify the genetic control of heading time in wheat, and the following three component characters have been identified: vernalization requirement, photoperiodic sensitivity and narrow-sense earliness (earliness per se). Vernalization requirement means the sensitivity of the plant to low temperature for accelerating spike primordium formation, and vernalization insensitivity (spring habit) genes, Vrn-A1, Vrn-B1, and Vrn-D1 (previously known as Vrn1, Vrn2, and Vrn3) were genetically identified on chromosomes 5A, 5B, and 5D, respectively (Flood and Halloran 1986, for review). The photoperiodic (long-day) response is determined by dominant genes, Ppd-A1, Ppd-B1, and Ppd-D1 (formerly Ppd3, Ppd2, and Ppd1), which control the sensitivity to photoperiod, and are located on chromosomes 2A, 2B, and 2D, respectively (Worland 1996, for review). Narrow-sense earliness or earliness per se is the earliness of fully vernalized plants grown under long days, and no major genes have been detected for this character.
Over the past decade, extensive studies in Arabidopsis have revealed the genetic and molecular mechanisms of the floral transition from vegetative growth to flowering (Boss et al. 2004, for review). According to the current understanding in Arabidopsis, there are four major flower-promoting pathways. The vernalization and photoperiod pathways integrate environmental signals into the floral phase transition, whereas the autonomous and gibberellin (GA) pathways act independently of external stimuli. The vernalization pathway mediates the promotion of floral transition induced by low temperatures. FLOWERING LOCUS C (FLC), a MADS box gene acting as repressor of floral transition, is regulated negatively by vernalization but positively by FRlGlDA (FRI). FRI encodes a novel protein containing two-coiled coil domains, indicating that it interacts with other proteins or nucleic acids. Vernalization results in the stable reduction of the levels of flowering repressor FLC by the epigenetic regulation. Recent studies indicate that activation and repression of FLC expression is regulated by chromatin modification (He and Amasino 2005, for review). VERNALIZATION INSENSITIVE 3 (VIN3) is responsible for the initial repression of FLC during exposure to cold (vernalization). VIN3 encodes a PHD (plant homeodomain) - finger-containing protein, which is involved in the initiation of the vernalization-mediated histone modifications. Furthermore, the stable silencing of FLC locus requires the plant-specific DNA binding protein VRN1, the product of VERNALIZATION 1, and the polycomb-group protein VRN2 from VRN2 gene. The photoperiod pathway promotes the floral transition in response to long days, which consists of photoreceptor, circadian clock and circadian clock-regulated genes (Searle and Coupland 2004, for review). The light signal perceived by photoreceptors such as phytochrome (PHY) and cryptochrome (CRY) synchronizes the circadian clock to the seasonal changes in day length. CIRCADIAN CLOCK ASSOCIATED 1 (CCA1), LATE ELONGATED HYPOCOTYL (LHY) and TIMING OF CAB (chlorophyll a/b binding protein) EXPRESSION 1 (TOC1) have been identified as oscillator components of circadian rhythms. CCA1 and LHY are proteins similar to a single MYB repeat. TOC1 has an N-terminus similar to the receiver domain of bacterial response regulators and C-terminus with the plant-specific CCT domain. Circadian clock -regulated genes are GIGANTEA (GI), FLAVIN-BINDING, KELCH REPEAT, F-BOX (FKF1), CONS TANS (CO) and FLOWERING LOCUS T (FT). GI is a nuclear protein whose function is unknown, but is presumed to maintain the viability of the circadian system. FKF1 protein functions as blue-light receptor and increases the CO mRNA level (Hayama and Coupland 2004, for review). FKF1 contains F-box and kelch motif repeat as well as a LOV domain of blue-light receptor, suggesting that this protein is associated with degradation of other protein such as specific transcription factor of CO. Both mRNA and protein level of FKF1 oscillate, indicating that this gene acts as clock -output. CO encodes a transcription factor with two B-box zinc fingers and directly induces of FT expression. In long-day conditions, CO expression coincides with the light input resulting in the activation of FT expression. FT encodes a protein that is similar to animal Raf kinase inhibitors and is highly homologous to flowering repressor TERMINAL FLOWER 1 (TFL1). Contrary to the flower-promoting activity of FT, TFL1 functions as a flowering repressor. It is unknown how they have opposite functions in spite of their common structure. In addition to environmental factors such as temperature and photoperiod, internal signals also regulate flowering. The genes of autonomous pathway prevent the accumulation of mRNA of FLC by epigenetic and post-transcriptional regulation (Simpson 2004, for review). The autonomous pathway comprises at least six genes, FCA, FY, FPA, FVE, LD (LUMINIDEPENDENS), and FLD (FLOWERING LOCUS D), mutation of which produces the late-flowering phenotype. FCA encodes an RNA-binding protein, and FY encodes a WD-repeat protein, the yeast homolog of which is involved in RNA 3' end processing. The current model is that the FCA-FY complex functions in a negative feedback loop that limits the amount of functional FCA protein (Putterill et al. 2004, for review). FPA and FVE also encode an RNA-binding protein and a WD-repeat protein, respectively. LD encodes a homeodomain protein that might function in RNA processing. FLD encodes a protein similar to a component of mammalian histone deacetylase complexes, indicating that FLD acts to deacetylate FLC chromatine and prevent FLC expression. Genes involved in the autonomous pathway such as FCA also negatively regulate FLC expression, indicating that the vernalization and autonomous pathways are connected in the FLC gene. These two pathways commonly promote floral transition by reducing the level of FLC expression, which then promote the expression of SUPPRESSOR OF OVEREXPRESSION OF CO1 (SOC1). The growth regulator GA promotes flowering by up-regulating the transcription level of a floral meristem identity gene LEAFY (LFY). FT and SOC1 in turn activate floral meristem identity gene APETALA1 (AP1) which promotes the formation of floral organs.
Recent progress in genomic comparison between Arabidopsis and rice revealed that the proteins involved in the photoperiod pathway are conserved in both species but the regulatory mechanisms for the photoperiodic response of flowering is diverged (Hayama and Coupland 2004, for review). For example, the rice genome contains the Co ortholog Hd1 (Heading date 1) and FT orthologHd3a. As mentioned above, CO induces FT expression under long-day conditions and promotes flowering in Arabidopsis. By contrast, Hd1 suppresses Hd3a and inhibits flowering under long-day conditions in the short-day plant rice. The difference in the molecular mechanism of FT/Hd3a regulation by CO/Hd1 between Arabidopsis and rice is not yet clear. The molecular studies in other plant species such as wheat may help us to understand how the diversity in the flowering pathway was generated during the evolution. This review focuses on recent advances we have made in understanding the molecular mechanism of floral transition in wheat.
WAP1 is an activator in vernalization pathway of floral transition in wheat
Among the genes involved in the floral transition in Arabidopsis, FLC, SOC1, and AP1 are members of the MADS box gene family encoding a large family of transcription factors (Riechmann and Meyerowitz 1997, for review). In addition to FLC, SOC1, and AP1, several MADS box genes were also identified to regulate flowering time in Arabidopsis; SHORT VEGETATIVE PHASE (SVP), CAULIFLOWER (CAL), FRUITFULL (FUL), and FLOWERING LOCUS M (FLM). FLM is also referred to as MADS AFFECTING FLOWERING1 (MAF1), a member of five FLC paralogs involved in the vernalization response. These facts indicate that the MADS box gene family plays important roles in many steps of the phase transition from vegetative growth to flowering.
To investigate the function of wheat MADS box genes in phase transition from vegetative to reproductive growth, we first cloned WAP1 (wheat AP1, formerly TaMADS#11, DDBJ accession no. AB007504) by screening a cDNA library from young spikes of common wheat cv. Norin 26 using the degenerate PCR products of genomic DNA corresponding to the MADS box region as probes (Murai et al. 1998, 2002). The deduced amino acid sequence of WAP1 revealed that 244 amino acid residues are highly homologous to that of AP1 (Murai et al. 2003). A phylogenetic study of the AP1-like MADS box genes indicated that WAP1 belongs to one subclade together with TaVRT-1 of wheat (Danyluk et al. 2003), VRN1 (unrelated to the gene with similar name in Arabidopsis) of T. monococcum (Yan et al. 2003), BM5 of barley (Schmitzet al. 2000), LtMADS1 of Lolium temulentum (Gocal et al. 2001), and RAP1B of rice (Kyozuka et al. 2000). In addition to a serine-rich motif and LPPWMLSHL/IN sequence in the C -terminal region, high sequence similarity among these genes throughout the coding region suggests that they are all orthologous genes. WAP1 is 98.8% identical to TaVRT-1 and has only three amino acid changes in the C region. WAP1 was isolated from spring wheat cv. Norin 26 (Murai et al. 1998, 2002), whereas TaVRT-1 from winter wheat cv. Fredrick (Danyluk et al. 2003), suggesting that they are the same gene and the difference in sequence is due to varietal polymorphism.
A transgenic study was performed using wheat cv. Akadaruma to identify the function of WAP1 in the floral transition (Murai et al. 2003). 'Akadaruma' wheat, which has none of the dominant alleles Vrn-A1, Vrn-B1 and Vrn-D1, shows a winter habit. Before the transformation study, we examined the expression pattern of WAP1 in the non-transformed 'Akadaruma' wheat. To examine the effects of developmental stage on the level of WAN mRNA, we performed RT-PCR analyses using the non-vernalized plants. The transcripts of WAP1 were not observed in an early stage and were increased significantly around the 5 to 7-leaf stage, when the growth phase changed from vegetative to reproductive, namely double-ridge stage. This is consistent with the report by Danyluk et al. (2003) that TaVRT - 1 expression was associated with the timing of the double-ridge formation. The effect of vernalization on the WAP1 expression was also determined. Sprouted seeds were cold- treated for 0 to 42 days, and then transferred to a growth chamber at 20°C. In the plants at the 3-leaf stage, in which mRNA of WAP1 is not detected under the non- vernalized condition, the WAP1 mRNA was clearly detected after 21 days of cold treatment. A high level of WAP1 expression was maintained in the growth chamber at 20°C, indicating that WAP1 is not just a cold stress-induced gene. These results suggest that the expression of WAP1 is associated with the floral transition The expression pattern of WAP1 that is up- regulated by vernalization was confirmed by Trevaskis et al. (2003).
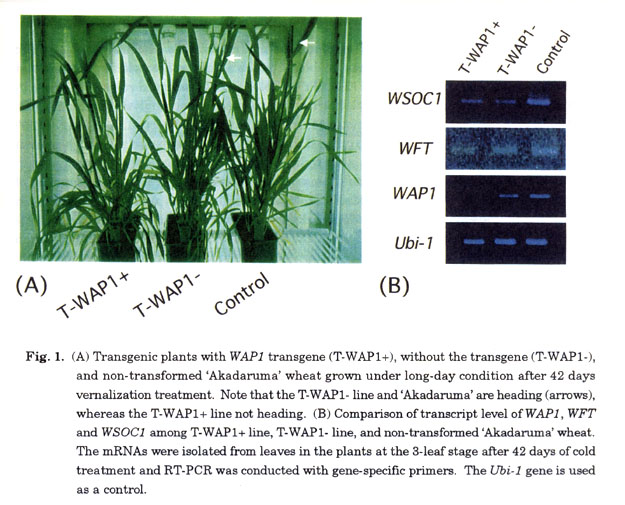
The WAP1 cDNA driven by rice actin 1 gene (Act1) promoter was introduced into wheat through particle bombardment, and transformants of wheat with the WAP1 transgene were produced (Mural et al. 2003). The T2 lines of two T1 plants with the WAP1 transgene homozygously (T-WAP1+ line) were used to examine the heading characters in a growth chamber. A non-transformed 'Akadaruma' wheat and null-segregant T2 lines of two T1 plants (T-WAP 1-line), which were not taken over the WAP1 transgene, were used as the controls. Sprouted seeds were exposed to 4°C for 42 days (vernalization), and then grown in a growth chamber under a 24-h day length regime at 20°C. T-WAP1+ lines headed later than T-WAP1- lines and control "Akadaruma" (Fig. 1). RT-PCR using the plants at the 3- leaf stage after a 42-day cold treatment revealed that the WAP1 expression was co- suppressed by WAP1 tranagene in the T-WAP1+ lines. The WAP1 transcripts were slightly detected in the T-WAP1+ line after an increase of PCR cycles, indicating that the co- suppression of WAP1 was incomplete and the level of gene expression was decreased in comparison with the control. Inhibition of the WAP1 expression in transgenic plants by co- suppression led to delay of heading in T-WAP1+ lines, clearly demonstrating that WAP1 acts as an activator in the vernalization pathway of the floral transition in wheat.
WAP1 is identical to Vrn gene for vernalization insensitiveness
Bread wheat is a hexaploid species with genome constitution AABBDD, which originated from three diploid relative species: A genome from T. urartu, B genome from Ae. speltoides or other species classified into Sitopsis section, and D genome from Ae. tauschii. Allopolyploidization leads to generating duplicated homoeologous genes, which is opposed to paralogous genes. Consequently, the hexaploid wheat genome contains triplicated homoeologous genes derived from the three diploid ancestors. Southern blot analysis with nulli-tetrasomic lines indicated that WAP1 has three homoeologous genes located on group 5 chromosomes, 5A, 5B, and 5D (Murai et al. 2003). Consequently, in the wheat genome there are three homoeologous WAP1 genes located on the group 5 chromosomes. Three cDNAs corresponding to three homoeologous genes of WAP1 were cloned, which are more than 97% identical with each other in amino acid sequence (unpublished data).
Yan et al. (2003) reported that VRN1, an orthologous gene of Vrn-A1 on 5A chromosome of bread wheat, is APi-like gene located on 5Am chromosome of diploid wheat T. monococcum. WAP1 is 98% identical to VRN1 and has only five amino acid changes. As mentioned above, WAP1 has three homoeologous genes located on group 5 chromosomes in bread wheat. These findings strongly suggest that WAP1 is ortholog of VRN1, and consequently three homoeologous genes of WAP1 located on 5A, 5B, and 5D chromosomes correspond to Vrn-A1, Vrn-B1, and Vrn-D1 genes, respectively.
We carried out expression studies using near-isogenic lines (NILs) for Vrn genes in wheat cv. Triple Dirk (TD) to examine the up-regulation of WAP1 by vernalization. The NILs with Vrn-A1, Vrn-B1 or Vrn-D1 dominant allele showed early-heading without vernalization, indicating that these Vrn genes activate the phase transition in non-vernalized plants. The NIL with all recessive alleles of Vrn genes delays heading under non-vernalization condition. Cold treatment for 35 days promoted heading in the NIL with all recessive alleles, in which the expression of WAP1 was induced by the cold treatment. Contrary to the expression pattern in the NIL with all recessive alleles, the expression of WAP1was slightly detected in the non-vernalized plants of NILs with Vrn dominant allele, and the level of expression was greatly increased by a short cold treatment. These observations are not contradictory to the idea that WAP1 is identical to the Vrn gene.
How is WAP1 (= Vrn genes) up-regulated by vernalization? In diploid wheat, Dubcovsky et al. (1998) identified a second gene affecting the response to vernalization and named it Vrn-Am2 (renamed VRN2 now). Contrary to the VRN1 dominant allele for spring growth habit, VRN2 allele for winter growth habit is dominant (Tranquilli and Dubcovsky 2000). The effect of VRN1 on heading time was significant only when the dominant VRN2 allele was present, that is, VRN2 is epistatic to VRN1. Yan et al. (2003) proposed a model of the vernalization pathway in diploid wheat according to the knowledge of the epistatic interactions between VRN1 and VRN2 and other available results. In their model, VRN2 encodes a putative repressor of VRN1 expression, which binds to the regulator region of the VRN1 gene. Later,VRN2 was identified by map-based cloning in diploid wheat (Yan et al. 2004). VRN2 encodes a protein with CTT motif that was identified in CO-like gene family and down-regulated by vernalization. As the vernalization process reduces the abundance of the VRN2 gene products, VRN1 transcription gradually increases, leading to the competence to flower. Recently, Fu et al. (2005) reported that the large deletions within the first intron in VRN1 gene are associated with spring growth habit in barley and wheat. However biochemical basis of the repression of VRN1 genes by VRN2 is still unknown. Does the VRN2 gene also act in bread wheat? It is interesting that the VRN2 locus has not been identified in bread wheat. It is possible that in hexaploid wheat there are three homoeologous orthologs of VRN2 with redundant functions, resulting in an unlikely event of finding a mutant phenotype. Assuming that the expression of WAP1 is repressed by VRN2 orthologs strongly in urn recessive allele and weakly in Vrn dominant allele in bread wheat, we can explain why WAP1 is up-regulated by vernalization in the spring habit NIL as well as in the winter habit NIL
WAP1 is also involved in photoperiod pathway
Wheat is a quantitative long-day plant, and short-day conditions delay the heading time. Ppd gene acts to reduce the delay of heading under short-day conditions. Our expression study of WAP1 with NILs for the Ppd gene in plants grown under different photoperiodic conditions indicated that both NILs with and without the Ppd gene dominant allele expressed WAP1 under the long-day condition, but not under the short-day condition (Murai et al. 2003). This demonstrates that WAP1 is up-regulated by a long photoperiod, and WAP1 and Ppd genes act on different pathways in promoting the floral transition. Our study revealed that WAP1 is expressed just before the floral transition, and is up-regulated by vernalization and long-photoperiod (Mural et al. 2003). Together with these findings, our transgenic study indicated that the WAP1 gene products act as an integrative activator in the down-stream of vernalization and photoperiod pathways of floral transition in wheat. Consequently, heading time of wheat clearly is determined by the expression of WAP1.
WFT, a gene located in up-stream of WAP1
In Arabidopsis , FT, SOC1, and LFY act as important integrators of multiple pathways that promote flowering, and activate the floral meristem identity gene AP1. Rice ortholog of FT was identified as flowering-time QTL named Hd3a. Transgenic rice over-expressing Hd3a gene is early flowering, indicating that Hd3a functions as a flowering promoter in rice as FT in Arabidopsis (Hayama and Coupland 2004, for review). Although the relationship between Hd3a and rice AP1 ortholog is unknown, we hypothesized that wheat FT ortholog is located up-stream of WAP1. Recently, we identified an FT homolog in wheat based on ESTs database (Ogihara et al. 2003), and named the gene WFT (Wheat FT). WFT has a PEBP (phosphatidyl ethanolamine-binding protein)-motif and has high homology to FT and Hd3a in the amino acid sequence (unpublished data). Our preliminary studies indicated that WFT expression is detected preceding the WAP1 expression in floral transition, and is maintained during the reproductive growth. WFT is up-regulated by long-day photoperiod. Furthermore, WFT expression is observed in the WAP1 co-suppression transgenic lines (Fig. 1). These findings suggest that WFT acts up-stream of the WAP1-related photoperiod pathway. Surprisingly, WFT is also up-regulated by vernalization. A transgenic study is indispensable for examining the function of WFT more precisely. Since ten FT-like genes were identified in the rice genome (Izawa et al. 2003, for review), there seems to be an FT-like gene family in wheat genome. It is necessary to identify other FT-like genes for understanding the flowering control pathway in wheat.
WSOC1, another activator of floral transition
To examine the function of SOC1 ortholog in wheat, we isolated WSOC1 (Wheat SOC1) from a cDNA library from wheat young spike (unpublished data). The expression analysis using WAP1 transgenic lines showed that WSOC1 was expressed in the WAP1 co-suppression lines as well as in control lines, indicating that WSOC1 is located up-stream to WAP1 or in a pathway different from WAP1 (Fig. 1). As WSOC1 is expressed from the early seedling stage and is induced by GA, we now hypothesize that WSOC1 acts as an activator of flowering in a pathway different from the WAP1-related pathway. A rice SOC1 ortholog, OsSOC1/ OsMADS5O, also functions as flowering activator (Lee et at. 2004; Andersen et at. 2004, for review). However, the relationships between OsSOC1/OsMADS5O and other genes for the flowering pathway in rice are unknown.
WFL is associated with spike formation not floral transition
FLORICAULA (FLO) of Antirrhinum and LFY of Arabidopsis encode plant-specific transcription factors and play major roles in the floral transition and floral meristem identity. In maize, two duplicated FLO/LFY orthologs play conserved roles with those in the dicot species (Bomblies et al. 2003). However, the expression analysis of rice FLO/LFY ortholog RFL suggested that the function of the RFL protein is diverged from that of LFY/ FLO (Kyozuka et al. 1998). RFL is expressed in all layers of young panicles but is absent in the primary branch differentiation sites, indicating that RFL is somehow associated with branching of lateral organs in the inflorescence meristem. To understand the function of FLO/LFY ortholog in wheat, we isolated WFL (Wheat FLO/LFY) and analyzed its expression pattern (unpublished data). In the double ridge stage, the WFL mRNA accumulation was localized in the lower ridge but absent in the upper ridge, where the spikelet primordia was being initiated. This expression pattern is very similar to that of RFL in rice young panicle, assuming that spikelet in wheat and primary branch in rice are homeotic lateral organs formed from inflorescence meristem. The WFL expression pattern indicates that WFL is associated with spikelet formation rather than floral meristem identity. To understand the functions of WFL, we need to obtain transgenic plants with loss-of-function or gain-of-function.
A model of the regulation of the floral transition in bread wheat
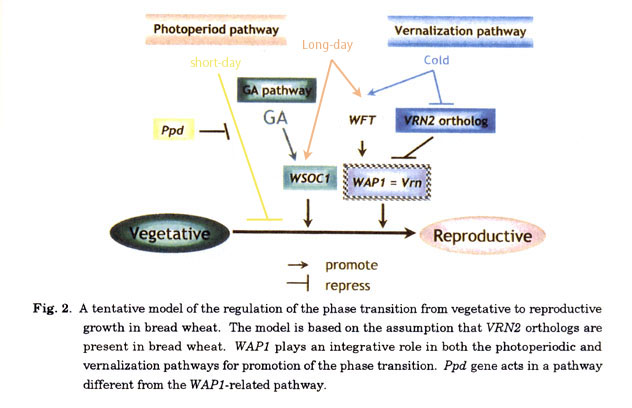
Fig. 2 shows a tentative model of the regulation of floral transition in bread wheat. The model is based on the assumption that VRN2 orthologs are present in bread wheat. In this model, WAP1 (= Vrn genes) plays a key role in the control of floral transition, which is up regulated by vernalization and long-days. In winter-habit cultivars with vrn recessive alleles, the expression of WAP1 is repressed by the VRN2 orthologs. Vernalization increased the expression of WAP1 by the depression of the products of VRN2 orthologs. In spring habit cultivars with Vrn dominant alleles, the VRN2 orthologs do not perfectly repress the expression of WAP1. In photoperiod pathway, WAP1 is up-regulated by WFT, which is activated by long-days. Our recent preliminary study indicated that WFT is also up regulated by vernalization. The relationship between the WFT-related pathway and putative VRN2 -related pathway in the response to vernalization is unknown. Our recent study also indicates that WSOC1 is associated with GA pathway, which is different from the WAP1 -related pathway. In conclusion, WAP1 is the principal factor that integrates the photoperiod and vernalization pathways, and the WAP1 expression determines the heading time in wheat. The Ppd gene that prevents the delay of heading under short-day condition is involved in the different pathways from the WAP1 -related pathway. In Fig. 2, we can not find the major repression pathway of floral transition in wheat except for Ppd -related minor pathway in the photoperiod pathway. A flowering repressor FLC is the most important regulator of floral transition in Arabidopsis, which integrates autonomous and vernalization pathways (Boss et al. 2004; Amasino 2004, both for review). Why orthologs of FLC have not been found in the genomes of wheat and other cereals remains a mystery. Other repressor may exist in cereal genomes. The present model of the regulation of the floral transition in wheat should be modified when cloning and analyses of novel genes are achieved in future.
Acknowledgements
This work was conducted with the support of all members of the Murai's laboratory in the Department of Bioscience, Fukui Prefectural University, Japan.
References
Amasino R (2004) Vernalization, competence, and the epinegetic memory of winter. Plant Cell 16: 2553-2559.
Andersen CH, Jensen CS and Petersen K (2004) Similar genetic switch systems might integrate the floral inductive pathways in dicots and monocots. Trends Plant Sci 9: 105-107.
Bomblies K, Wang R-L, Ambrose BA, Schmidt RJ, Meeley RB and Doebley J (2003) Duplicat FLORICAULA/LEAFY homologs zfl1 and zfl2 control inflorescence architecture and flower patterning in maize. Development 130: 2385-2395.
Boss PK, Bastow RM, Mylne JS and Dean C (2004) Multiple pathways in the decision to flower: enabling, promoting, and resetting. Plant Cell 16: S18-S31.
Danyluk J, Kane NA, Breton G, Limin AE, Fowler DB and Sarhan F (2003) TaVRT-1, a putative transcription-factor associated with vegetative to reproductive transition in cereals. Plant Physiol 139:1849-1860.
Dubcovsky J, Lijavetzkyu D, Appendino L and Tranquilli G (1998) Comparative RFLP mapping of Triticum monococcum genes controlling vernalization requirement. Theor Appl Genet 97: 968 975.
Flood RG, Halloran GM (1986) Genetics and physiology of vernalization response in wheat. Adv Agr 39:87-125.
Fu D, Szucs P, Yan L, Helguera M, Skinner JS, von Zitzewitz J, Hayes PM and Dubcovsky J (2005) Large deletions within the first intron in VRN-1 are associated with spring growth habit in barley and wheat. Mol Genet Genomics 273: 54-65.
Gocal GFW, King RW, Blundell CA, Schwartz OM, Andersen CH and Weigel D (2001) Evolution of floral meristem identity genes. Analysis of Lolium temulentum genes related to APETALA1 and LEAFY of Arabidopsis. Plant Physiol 125: 1788-1780.
Hayama R and Coupland G (2004) The molecular basis of diversity in the photoperiodic flowering responses of Arabidopsis and rice. Plant Physiol 135: 677-684.
He Y and Amasino RM (2005) Role of chromatin modification in flowering-time control. Trends Plant Sci 10: 30-35.
Izawa T, Takahashi Y and Yano M (2003) Comparative biology comes into bloom: genomic and genetic comparison of flowering pathways in rice and Arabidopsis. Curr Opin Plant Biol. 6: 113-120.
Kyozuka J, Konishi S, Nemoto K, Izawa T and Shimamoto K (1998) Down-regulation of RFL, the FLO /LFY homolog of rice, accompanied with panicle branch initiation. Proc Natl Acad Sci USA 95: 1979-82.
Kyozuka J, Kobayashi T, Morita M and Shimamoto K (2000) Spatially and temporally regulated expression of rice MADS box genes with similarity to Arabidopsis class A, B and C genes. Plant Cell Physiol 41: 710-718.
Lee S, Kim J, Han J-J, Han M-J and An G (2004) Functional analyses of the flowering time gene OsMADS5O, the putative SUPPRESSOR OF OVEREXPRESSION OF CO1/AGAMOUS-LIKE2O (SOC1/AGL2O) ortholog in rice. Plant J 38: 754-764.
Murai K, Murai R, Takumi S and Ogihara Y (1998) Cloning and characterization of cDNAs corresponding to the wheat MADS box genes. Proc IX Int Wheat Genet Symp Saskatoon 1: 89 94.
Murai K, Takumi S, Koga H and Ogihara Y (2002) Pistillody, homeotic transformation of stamens into pistil-like structures, caused by nuclear-cytoplasm interaction in wheat. Plant J 29: 169 181.
Murai K, Miyamae M, Kato H, Takumi S and Ogihara Y (2003) WAP1, a wheat APETALA1 homolog, plays a central role in the phase transition from vegetative to reproductive growth. Plant Cell Physiol 44: 1255-1265.
Ogihara Y, Mochida K, Nemoto Y, Murai K, Yamazaki Y, Shin-I T and Kohara Y (2003) Correlated clustering and virtual display of gene expression patterns in the wheat life cycle by large-scale statistical analyses of expressed sequence tags. Plant J 33: 1001-1011.
Putterill J, Laurie R and Macknight R (2004) It's time to flower: the genetic control of flowering time. BioEssays 26: 363-373.
Riechmann JL and Meyerowitz EM (1997) MADS domain proteins in plant development. Biol Chem 378:1079-1101.
Schmitz J, Franzen R, Ngyuen TH, Garcia-Maroto F, Pozzi C, Salamini F and Rohde W (2000) Cloning, mapping and expression analysis of barley MADS-box genes. Plant Mol Biol 42: 899-913.
Searle I and Coupland G (2004) Induction of flowering by seasonal changes in photoperiod. EMBO J 23: 1217-1222.
Simpson GG (2004) The autonomous pathway: epigenetic and post-transcriptional gene regulation in the control of Arabidopsis flowering time. Curr Opi Plant Biol 7: 570-574.
Tranquilli G and Dubcovsky J (2000) Epistatic interaction between vernalization genes VrnAm1 and Vrn-Am2 in diploid wheat. J Heredity 91: 304-306.
Trevaskis B, Bagnall DJ, Ellis MI!, Peacock WJ and Dennis ES (2003) MADS box genes control vernalization-induced flowering in cereals. Proc Natl Acad Sci USA 100: 13099-13104.
Yan L, Loukoianov A, Tranquilli G, Helguera M, Fahima T and Dubcovsky J (2003) Positional cloning of the wheat vernalization gene VRN1. Proc Natl Acad Sci USA 100: 6263-6268.
Yan L, Loukoianov A, Blechl A, Tranquilli G, Ramakrishna W, SanMiguel P, Bennetzen JL, Echenique V and Dubcovsky J (2004) The wheat VRN2 gene is a flowering repressor down-regulated by vernalization. Science 303: 1640-1644.
Worland AJ (1996) The influence of flowering time genes on environmental adaptability in European wheats. Euphytica 89:49-57.