Memorial Issue, Wheat Information Service No.100
Genetic regulation of inflorescence architecture: common themes and divergent pathways in Arabidopsis and cereal crops
Mary E. Byrne
Department of Crop Genetics, John lnnes Centre, Norwich NR4 7UH, UK
E-mail address: mary.byme@bbsrc.ac.uk
Key words: wheat, maize, rice, Arabidopsis, meristem, inflorescence
Introduction
The discovery of the structure of DNA and its publication in 1953 (Watson and Crick 1953) marks a turning point in biological sciences. The 50 years since has witnessed quantum advances in our understanding and knowledge of the basic unit of hereditary extending from determining the genetic code to determining the entire sequence of the human genome. The greatest impact on plant sciences has been driven by use of the model species Arabidopsis thaliana. Development of substantial genetic and genomic resources in Arabidopsis is underpinning a rapidly expanding knowledge of plant biology. Arabidopsis has been and will continue to be the most tractable system for addressing many fundamental questions and will provide a useful tool for defining gene function and establishing genetic pathways.
However, a key challenge in developmental biology is identifying genes associated with generating diversity of form. Although some genetic pathways and the corresponding genes in these pathways will be highly conserved throughout land plants, it is also clear that others have diverged to a lesser or greater extent. This is likely the result of small scale changes that affect gene expression patterns or protein function, as well as large-scale changes involving whole genome duplication. Genome duplication has occurred repeatedly throughout the evolution of most higher plant lineages (Paterson et al. 2004; Taylor and Raes 2004). Many genes are lost following such duplications. However, duplicate genes may be retained by selection for functional divergence. One such process is subfunctionalizatjon, where the products of a duplication retain some but not all of the original gene function. Duplicate genes may also be retained through neofunctionalization. In this case one of the duplicate genes acquires a new, potentially adaptive function. To understand phenotypic variation it is important then to characterize genetic regulatory pathways in a diverse range of plant species.
Arabidopsis is a member of a major group of higher plants, known as dicotyledons that produce two seed leaves. A second major higher plant group, the monocotyledons, are characterized by a single seed leaf. The monocotyledons, which include the grass family Poaceae, are estimated to have diverged from dicotyledons approximately 200 million years ago (Wolfe et al. 1989). Since this divergence plant lineages within both groups have undergone large-scale genome duplications such that a comparison of genes between monocots and dicots is predicted to reveal many cases of subfunctionalization and acquired new gene function.
The Poaceae are of special interest as some of the world's major crops, including rice, maize, wheat and barley, are grass species. Until recently these species were less tractable for molecular genetic studies due to relatively large diploid genome sizes and, in the case of bread wheat, due to polyploidy. However, technological developments and reduced costs have meant that it is now feasible to develop large-scale genomics resources in other species. The past few years has seen a rapid expansion in this area in grass species with completion of the rice genome sequence (Goff et al. 2002; Yu et al. 2002), and a large proportion of gene sequence in maize, as well as sorghum (Martienssen et al. 2004; Bedell et al. 2005). These resources provide the opportunity for fully exploring genetic regulation of biological processes in crop species. The information obtained will also impact on comparative genomics as well as evolutionary genetics, defining conserved and divergent pathways.
In this review I will compare and contrast genetic regulation of inflorescence development in the dicot species Arabidopsis and Antirrhinum with our current knowledge of genes controlling inflorescence development in the key grass crop species maize, rice, wheat and barley. Several examples will be used to highlight the role of meristems in determining inflorescence architecture.
Meristems and plant development
The plant life cycle is an alternation between haploid and diploid phases with a predominant diploid sporophytic stage resulting from union of female and male haploid gametophytes. Subsequent to fertilization, development of the embryo starts e with a single cell that undergoes successive rounds of cell divisions to produce a sphere of cells, or globular embryo. Patterning of the embryo occurs at this early stage. Cell layers in a radial dimension as well as apical and basal regions of the embryo can be distinguished. The apical region forms the cotyledons and a defined structure, the shoot apical meristem. Throughout the plant life cycle the shoot apical meristem remains at the apex of the plant (Fig. 1), and gives rise to all aerial portions of the plant including leaves and stems, as well as secondary axillary meristems that form branches and flowers. Within the basal region of the embryo a similar structure, the root apical meristem, is established and this meristem ultimately gives rise to the primary root system of the plant.
In plants then, most organs are specified postembyonically, after germination of the embryo from the seed. This developmental habit is the result of establishment and maintenance of stem cell populations within the shoot and root meristems. Stem cells in the centre of these meristems are undifferentiated and slowly dividing. Divisions of these stem cells give rise to daughter cells some of which maintain the pool of stem cells and others contribute to the peripheral region of the meristem. Peripheral region cells divide more rapidly and the products of these cell divisions, on the flanks of the meristem, are recruited for differentiation. In the case of the shoot apical meristem cells on the flanks of the meristem are recruited as founder cells for organ formation. Subsequent to recruitment cells in developing lateral organs further divide, expand and terminally differentiate, and so contribute to mature organs of the plant body.

Genetic pathways regulating shoot meristem function
The organization of the shoot apical meristem of higher order plants has been defined classically by histology and more recently by gene expression patterns. Typically discrete cell layers can be distinguished. The outer layer (L1) gives rise mainly to outermost epidermal tissues. Either one or two inner cell layers (L2 and L3) contribute to internal ground tissue and vasculature of mature organs. Superimposed on this organization is a central zone of highly vacuolated and slowly dividing cells that are readily distinguished from more rapidly dividing, cytoplasmically dense peripheral zone cells. Cells on the margins of the peripheral zone are recruited into differentiating structures. Clonal analysis provides evidence that a small number of stem cells persist for some time at the apex contributing to many nodes through plant development (Tilney-Bassett 1986).
Studies in Arabidopsis have defined two major pathways that are involved in maintaining the shoot apical meristem. One pathway involves interactions between a homeodomain transcription factor WUSCHEL (WUS) and signaling components within the CLAVATA (CLV) complex. WUS is expressed very early in embryogenesis and is rapidly confined to a few cells in inner layers of the central zone (Mayer et al. 1998). Mutations in WUS result in termination of the shoot meristem and loss of stem cell function whereas overexpression results in proliferation of stem cells (Laux et al. 1996; Schoof et al. 2000). WUS acts in concert with CLV genes. CLV1 encodes a leucine-rich repeat transmembrane kinase expressed in a small group of cells immediately above the region of WUS expression (Clark et al. 1997). CLV1 interacts directly with another transmembrane leucine-rich repeat protein, CLV2 (Jeong et al. 1999). A third member of this pathway is CLV3, which encodes a secreted protein expressed in outer layers of the meristem in a domain partly overlapping that of CLV1 (Fletcher et al. 1999). CLV3 acts non-cell autonomously to regulate WUS expression and this is likely via interaction with CLV1 (Rojo et al. 2002; Lenhard and Laux 2003). Mutations in CLV genes result in an enlarged meristem and an expanded domain of WUS expression. Interactions between WUS and CLV form a positive feedback loop to maintain meristem homeostasis by maintaining a balanced population of stem cells in the central zone of the meristem (Brand et al. 2000; Schoof et al. 2000).
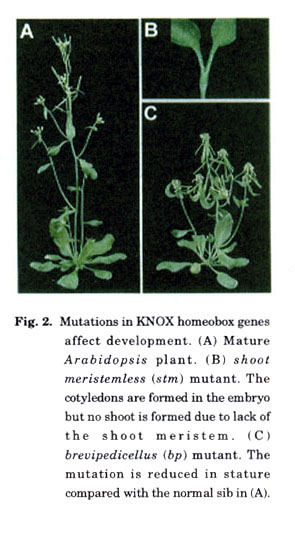
A second pathway required for maintenance of stem cell fate in the shoot meristem involves KNOX and BELL class genes, both of which belong to a larger group of TALE class homeodomain transcription factors. In Arabidopsis class 1 KNOX genes are primarily expressed in the shoot apical meristem and downregulated in differentiating cells. Mutations in the KNOX gene SHOOT MERISTEMLESS (STM) results in loss of meristem function (Fig. 2) (Barton and. Poethig 1993; Endrizzi et al. 1996; Long et al. 1996). One means by which STM maintains meristem function is via repression of differentiation program genes ASYMMETRIC LEA VES1 and ASYMMETRIC LEA VES2 (Byrne et al. 2000, 2002). The closest relative of STM in Arabidopsis is the KNOX gene BREVIPEDICELLUS (BP). Although these genes are closely related they have different expression patterns within the meristem and different roles in meristem function (Fig. 2). STM is expressed throughout the central and peripheral regions of the meristem, whereas BP is predominantly expressed in the peripheral region of the meristem (Lincoln et al. 1994; Long et al. 1996). BP is not essential for menstem function as bp mutants develop a shoot (Byrne et al. 2002; Douglas et al. 2002; Venglat et al. 2002). A predominant defect in bp mutants is reduced stature (Fig. 2). This phenotype coincides in part with the expression pattern of BP in cells of the meristem that ultimately contribute to internodes. Although BP has no direct role in maintaining the shoot meristem, BP is able to promote vegetative and inflorescence meristem function, but not floral meristem function, in the absence of STM (Byrne et al. 2002). However, this is apparent only in the absence of AS1 or AS2. Thus STM and BP are functionally redundant, but each gene has also acquired some degree of specialization. Two other class I KNOX genes in Arabidopsis, KNAT2 and KNAT6, are duplicate genes closely related to each other but more distantly related to STM and BP. Loss of KNAT2 has no phenotypic defects, indicating functional redundancy with KNAT6. The role of these genes in meristem function is yet to be determined (Byrne et al. 2002).
A more distantly related BELL class homeobox gene, BELLRINGER (BLR) is also expressed in the shoot meristem, in a pattern that overlaps with that of STM and BP. Mutations in BLR result in plants with reduced stature, increased leaf and flower number and altered arrangement of flowers on the plant stem. Although BLR by itself is not essential for meristem function, activities of STM and BP re dependant on BLR (Byrne et al. 2003; Smith and Hake 2003; Bhatt et al. 2004). KNOX and BELL class genes form a network of protein-protein interactions (Hackbusch et al. 2005) suggesting multiple levels of combinatorial interactions and potential redundancies function to pattern and maintain the shoot meristem.
Inflorescence development in dicot species Arabidopsis and Antirrihnum
Throughout the plant life cycle the shoot meristem undergoes a number of transitions. Subsequent to germination a vegetative phase of development ensues where the shoot meristem produces leaves (Fig. 1). On transition to reproductive development the shoot meristem forms an inflorescence meristem (Fig. 1). This transition is typically associated with changes in the size of the meristem and with changes in gene expression patterns. The inflorescence meristem can produce different types of secondary meristems or axillary meristems such as branch meristems or floral meristems that differentiate to form flowers. The type and pattern of axillary meristem produced by the inflorescence meristem greatly impacts the architecture of the inflorescence.
Arabidopsis transitions to reproductive development, in inductive conditions, after producing around 6-8 vegetative leaves. During the reproductive stage development is marked by a substantial increase in internode elongation, production of several cauline leaves followed by formation of an indeterminate number of flowers. Lateral branches, initiated in the axils of cauline and vegetative leaves, reiterate the development of the main axis. Mutations affecting meristem function at all phases of this developmental programme have been described. One example is TERMINAL FLOWER1 (TFL1) and its homolog CENTRORADIALIS (CEN) in the dicot Antirrhinum. TFL1 and CEN are required to maintain inflorescence meristem indeterminate identity. Mutations in TFL1 /CEN result in the conversion of the inflorescence into a terminal flower (Shannon and Meekswagner 1991; Alvarez et al. 1992; Bradley et al. 1996, 1997). In addition to its effect on meristem fate, TFL1 also extends the vegetative phase of Arabidopsis, but CEN does not seem to have a flowering time role in Antirrhinum (Shannon and Meekswagner 1991; Bradley et al. 1996).
Common themes and variation in grass inflorescence development
In contrast to Arabidopsis and Antirrhinum grasses are characterized by the spikelet, a reduced branch that gives rise to floral meristems. Spikelets may arise directly on the main inflorescence axis or from primary or secondary inflorescence axillary meristems. The degree and pattern of branching and specification of spikelet meristem identity within and between species determines spike architecture. This can be highlighted by comparison of spike development in several of the major cereal crops.
Domesticated maize is unusual amongst the grasses as it produces two types of inflorescences, the tassel and the ear. The tassel forms at the apex of the plant, whereas the ear forms in a lateral position from an axillary branch. Although the mature tassel and ear appear different, development is very similar. One primary difference is that the tassel produces branch meristems whereas the ear is unbranched. Branch meristems reiterate the pattern of development of the main inflorescence meristem in the tassel. Both tassel and ear produce three other meristems. Spikelet pair meristems each produce two spikelet meristems. These spikelet meristems in turn produce two floral meristems that give rise to florets or the grass flower. Alteration in the growth of these different meristems leads to variation in architecture between tassel and ear and between different maize inbred lines. Alteration in axillary meristem identity and determinacy also accounts for variation between inflorescence architecture between different grasses.
The inflorescence, or panicle, of rice is more branched than the tassel of maize. Axillary meristems arising from the main inflorescence meristem are branch meristems. These primary branch meristems initially produce secondary branch meristems and later in development form spikelet meristems. Spikelets on primary and secondary branches give rise to a single floral meristem. Both primary and secondary branch meristems are determinant and form a terminal spikelet.
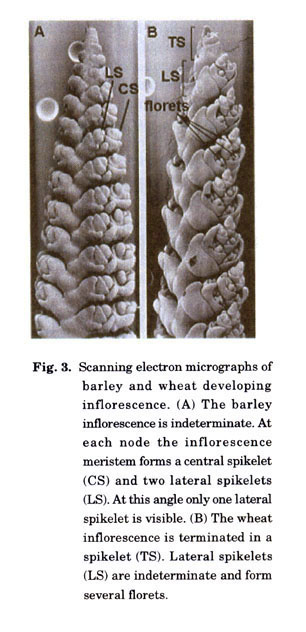
Wheat and barley are closely related members of the Triticeae within the family Pooideae and are only distantly related to rice and maize (Keller and Feuillet 2000; Kellogg 2001). Inflorescence development differs from that in maize and rice in that inflorescences, or spikes, are characteristically unbranched. In hexaploid wheat the main axis, or rachis, carries sessile spikelets consisting of 2 glumes and 2-5 florets arranged along an indeterminate axis (Fig. 3). The main axis in wheat is determinant and terminates with a terminal spikelet, although in the diploid T. monococcum the terminal spikelet is rudimentary, barren or missing (Bonnett 1936; Murai et al. 2002). Variation in the number of florets in a spike occurs principally within a spikelet. In barley three spikelets form along a main axis and each spikelet initiates one floral meristem and so forms one floret (Fig. 3). All three spikelets develop to maturity in 6-rowed barley whereas only the central spikelet develops in 2-rowed barley. In contrast to wheat, growth of the main axis is indeterminate and so can contribute to variation in the number of florets in a spike (Bonnett 1935; Babb and Muehlbauer 2003).
Regulators of inflorescence development in cereal grasses
Key features determining maize and rice inflorescence architecture are meristem identity and determinacy, and this is highlighted by several examples of mutants that affect these processes. Initiation of axillary meristems requires barren stalk1 (ba1) in maize and LAX PANICLE (LAX) in rice. ba1 mutants lack vegetative branches as well as ears and have unbranched tassels lacking spikelets (Gallavotti et al. 2004). In rice lax mutants, the number of primary branches and spikelets is also strongly reduced (Komatsu et al. 2001, 2003b). Both ba1 and LAX encode orthologous basic helix-loop-helix transcription factors. In maize early axillary meristems formed by the inflorescence meristem form branch meristems and later axillary meristems form spikelet pair meristems. Spikelet pair meristems in ramosa mutants are converted to branch meristems making many more branches with many spikelet meristems compared to the normal maize tassel (Veit et al. 1993). In maize there are genes such as branched silkless1 (bd1) required for the transition from spikelet to floret meristem identity (Colombo et al. 1998; Chuck et al. 2002). Spikelet meristems in bd1 mutants, instead of forming two florets, continue to produce spikelet pair or spikelet meristems. bd1 is an ERF transcription factor and close relatives have been identified in other grasses with branched inflorescences including rice, oat and sorghum (Chuck et al. 2002; Komatsu et al. 2003a). indeterminate spikelet (ids1), an AP2-like MADS box transcription factor, specifies spikelet meristem determinacy such that mutants form extra florets (Chuck et al. 1998).
Other genes that pattern inflorescence architecture are those required for meristem maintenance. Recessive mutations in the maize gene KNOTTED1, the founding member of the KNOX family of homeobox transcription factors, result in loss of the shoot apical meristem (Kerstetter et al. 1997; Vollbrecht et al. 2000). However, the severity of this phenotype is background dependant, indicating variable redundancy in this pathway. In weakly penetrant backgrounds the number of branches and spikelet pairs are reduced (Kerstetter et al. 1997).
fasciated earl (fea1) and thick tassel dwarf1 (td1) in maize are also required to maintain inflorescence meristem size. In fea1 mutants, meristem cell proliferation results in enlarged and fasciated inflorescence meristems and consequently an increase in seed row number in the ear and spikelet density in the tassel. fea1 encodes a leucine-rich repeat receptor like gene closely related to CLV2 in Arabidopsis (Taguchi-Shiobara et al. 2001). td1 also affects inflorescence meristem size and spikelet number in the ear and tassel. td1 encodes a putative leucine-rich repeat receptor-like kinase closely related to the Arabidopsis gene CLV1 (Bommert et al. 2005). In contrast to maize, the rice gene most closely related to CLV1, FLORAL ORGAN NUMBER1 (FON1), is only required to maintain floral meristem size (Suzaki et al. 2004). In fon1 mutants floral meristems are enlarged and there is an increase in floral organ number. The inflorescence meristem is not affected.
Models and crops: conservation and divergence
While inflorescence architecture of Arabidopsis and grasses vary significantly, comparable genetic pathways govern some processes. Conservation of function of KNOX transcription factors is indicated by kn1 and the Arabidopsis orthologue STM. Recessive mutations in both genes result in loss of shoot meristem maintenance, although as noted above the severity of meristem loss in maize is background dependent and likely to reflect variable degrees of redundancy (Long et al. 1996; Kerstetter et al. 1997; Vollbrecht et al. 2000). Meristem size in Arabidopsis is regulated in part by the CLAVATA gene complex, such that mutations in CLV genes result in enlargement of inflorescence and floral meristems (Fletcher 2002). In maize mutations in fea1 and td1, closest relatives of CLV2 and CLV1 respectively, result in enlarged menstems. However, in Arabidopsis CLV1 and CLV2 act in the same genetic pathway, whereas the combined effect of fea1 and td1 mutants results in extreme enlargement of meristems indicating these genes are not exclusively in the same pathway (Bommert et al. 2005). Despite these examples it is evident that some genes regulating inflorescence development will not be conserved between Arabidopsis and grasses. For instance, there is no dear Arabidopsis orthologue of the maize ba11 and rice LAX (Komatsu et al. 2003b; Gallavotti et al. 2004).
Two key challenges in developmental biology are identifying regulatory genes and genes associated with generating diversity of form within and between species. As we gain further knowledge of fundamental biology we can use this information to identify genes that potentially impact on crop improvement. This will be particularly relevant to isolating quantitative trait loci (QTL) and for use in association studies. As an example fea1 and td1 both map to yield quantitative trait loci in maize (Taguchi-Shiobara et al. 2001; Bommert et al. 2005). Accumulation of data from different species using a combination of forward and reverse genetics as well as genome synteny between grass species will contribute to a comprehensive model of evolution and domestication of inflorescence architecture in cereal grasses. This will in turn provide a resource for isolating quantitative yield traits, selecting improved variants of existing yield traits and for developing useful novel traits.
Acknowlegments
I would like to thank Lorelei Bilham for the SEM images of barley and wheat inforescences.
References
Alvarez J, Guli CL, Yu X-H and Smyth DR (1992) terminal flower: a gene affecting inflorescence development in Arabidopsis thaliana. Plant J 2: 103-116.
Babb S and Muehlbauer GJ (2003) Genetic and morphological characterization of the barley uniculm2 ( cul2 ) mutant. Theor Appl Genet 106: 846-857.
Barton MX and Poethig RS (1993) Formation of the shoot apical meristem in Arabidopsis thaliana - an analysis of development in the wild type and in the shoot meristemless mutant. Development 119:823-831.
Bedell JA, Budiman MA, Nunberg A, Citek RW, Robbins D, Jones J, Flick E, Rholfing T, Fries J, Bradford K, McMenamy J, Smith M, Holeman H, Roe BA, Wiley G, Korf IF, Rabinowicz PD, Lakey N, McCombie WR, Jeddeloh JA and Martienssen RA (2005) Sorghum genome sequencing by methylation filtration. PLoS Biol 3: e13.
Bhatt AM, Etchells JP, Canales C, Lagodienko A and Dickinson H (2004) VAAMANA-a BEL1-like homeodomain protein, interacts with KNOX proteins BP and STM and regulates inflorescence stem growth in Arabidopsis. Gene 328: 103-Co11.
Bommert P, Lunde C, Nardmann J, Vollbrecht E, Running M, Jackson D, Hake S and Werr W (2005) thick tassel dwarf1 encodes a putative maize ortholog of the Arabidopsis CLAVATA1 leucine - rich repeat receptor-like kinase. Development 132: 1235-1245.
Bonnett OT (1935) The development of the barley spike. J Agr Res 51:451-457.
Bonnett OT (1936) The development of the wheat spike. J Agr Res 53: 445-451.
Bradley D, Carpenter R, Copsey L, Vincent C, Rothatein S and Coen E (1996) Control of inflorescence architecture in Antirrhinum. Nature 379: 791-797.
Bradley D, Ratcliffe 0, Vincent C, Carpenter R and Coen E (1997) Inflorescence commitment and architecture in Arabidopsis. Science 275: 80-83.
Brand U, Fletcher JC, Hobe M, Meyerowitz EM and Simon R (2000) Dependence of stem cell fate in Arabidopsis on a feedback loop regulated by CLV3 activity. Science 289:617-619.
Byrne ME, Barley R, Curtis M, Arroyo JM, Dunham M, Hudson A and Martienssen RA (2000) Asymmetric leaves1 mediates leaf patterning and stem cell function in Arabidopsis. Nature 408: 967-971.
Byrne ME, Groover AT, Fontana JR and Martienssen RA (2003) Phyllotactic pattern and stem cell fate are determined by the Arabidopsis homeobox gene BELLRINGER. Development 130:3941- 3950.
Byrne ME, Simorowski J and Martienssen RA (2002) ASYMMETRIC LEAVES1 reveals knox gene redundancy. Development 129:1957-1965.
Chuck G, Meeley RB and Hake S (1998) The control of maize spikelet meristem identity by the APETALA2-like gene indeterminate spikelet1. Genes Devel 12: 1145-1154.
Chuck G, Muszynski M, Kellogg E, Hake S and Schmidt RJ (2002) The control of spikelet meristem identity by the branched silkless1 gene in maize. Science 298: 1238-1241.
Clark SE, Williams RW and Meyerowitz EM (1997) The CLAVATA1 gene encodes a putative receptor - kinase that controls shoot and floral meristem size in Arabidopsis. Cell 89: 575-585.
Colombo L, Marziani G, Masiero S, Wittich PE, Schmidt RJ, Gorla MS and Pe ME (1998) BRANCH SILKLESS mediates the transition from spikelet to floral meristem during Zea mays ear development. Plant J 16: 355-363.
Douglas SJ, Chuck G, Dengler RE, Pelecanda L and Riggs CD (2002) KNAT1 and ERECTA regulate inflorescence architecture in Arabidopsis. Plant Cell 14: 547-558.
Endrizzi K, Moussian B, Haecker A, Levin JZ and Laux T (1996) The SHOOT MERISTEMLESS gene is required for maintenance of undifferentiated cells in Arabidopsis shoot and floral meristems and acts at a different regulatory level than the meristem genes WUSCHEL and ZWILLE. Plant J 10: 101-113.
Fletcher JC (2002) Shoot and floral meristem maintenance in Arabidopsis. Annu Rev Plant Physiol Plant Mol Biol 53: 45-66.
Fletcher JC, Brand U, Running MP, Simon R and Meyerowitz EM (1999) Signaling of cell fate decisions by CLAVATA3 in Arabidopsis shoot meristems. Science 283: 1911-1914.
Gallavotti A, Zhao Q, Kyozuka J, Meeley RB, Ritter MX, Doebley JF, Pe ME and Schmidt RJ (2004) The role of barren stalk1 in the architecture of maize. Nature 432: 630-635.
Goff SA, Ricke D, Lan TH, Presting G, Wang R, Dunn M, Glazebrook J, Sessions A, Oeller P, Varma H, Hadley D, Hutchison D, Martin C, Katagiri F, Lange BM, Moughamer T, Xia Y, Budworth P, Zhong J, Miguel T, Paszkowski U, Zhang S, Colbert M, Sun WL, Chen L, Cooper B, Park S, Wood TC, Mao L, Quail P, Wing R, Dean R, Yu Y, Zharkikh A, Shen R, Sahasrabudhe S, Thomas A, Cannings R, Gutin A, Pruss D, Reid J, Tavtigian S, Mitchell J, Eldredge G, Scholl T, Miller RM, Bhatnagar S, Adey N, Rubano T, Tusneem N, Robinson R, Feldhaus J, Macalma T, Oliphant A and Briggs S (2002) A draft sequence of the rice genome (Oryza sativa L ssp.japonica). Science 296: 92-100.
Hackbusch J, Richter K, Muller J, Salamini F and Uhrig JF (2005) A central role of Arabidopsis thaliana ovate family proteins in networking and subcellular localization of 3-aa loop extension homeodomain proteins. Proc Natl Acad Sci USA 102:4908-4912.
Jeong S, Trotochaud AE and Clark SE (1999) The Arabidopsis CLAVATA2 gene encodes a receptor-like protein required for the stability of the CLAVATA1 receptor-like kinase. Plant Cell 11: 1925-1934.
Keller B and Feuillet C (2000) Colinearity and gene density in grass genomes Trends Plant Sci 5: 246-251.
Kellogg EA (2001) Evolutionary history of the grasses. Plant Physiol 125: 1198-1205.
Kerstetter RA, Laudencia-Chingcuanco D, Smith LG and Hake S (1997) Loss-of-function mutations in the maize homeobox gene, knotted1, are defective in shoot meristem maintenance. Development 124: 3045-3054.
Komatsu M, Chujo A, Nagato Y, Shimamoto K and Kyozuka J (2003a) FRIZZY PANICLE is required to prevent the formation of axillary meristems and to establish floral meristem identity in rice spikelets. Development 130: 3841-3850.
Komatsu M, Maekawa M, Shimamoto K and Kyozuka J (2001) The LAX1 and FRIZZY PANICLE 2 genes determine the inflorescence architecture of rice by controlling rachis-branch and spikelet development. Dev Biol 231: 364-373.
Komatsu K, Maekawa M, Ujiie S, Satake Y, Furutani I, Okamoto H, Shimamoto K and Kyozuka J (2003b) LAX and SPA: major regulators of shoot branching in rice. Proc Natl Acad Sci USA 100: 11765-11770.
Laux T, Mayer KFX, Berger J and Jurgens G (1996) The WUSCHEL gene is required for shoot and floral meristem integrity in Arabidopsis. Development 122: 87-96.
Lenhard M and Laux T (2003) Stem cell homeostasis in the Arabidopsis shoot meristem is regulated by intercellular movement of CLAVATA3 and its sequestration by CLAVATA1. Development 130: 3163-3173.
Lincoln C, Long J, Yamaguchi J, Serikawa K and Hake S (1994) A knotted1-like homeobox gene in Arabidopsis is expressed in the vegetative meristem and dramatically alters leaf morphology when overexpressed in transgenic plants. Plant Cell 6: 1859-1876.
Long JA, Moan El, Medford JI and Barton MK (1996) A member of the KNOTTED class of homeodomain proteins encoded by the STM gene of Arabidopsis. Nature 379: 66-69.
Martienssen RA, Rabinowicz PD, O'Shaughnessy A and McCombie WR (2004) Sequencing the maize genome. Curr Opin Plant Biol 7: 102-107.
Mayer KFX, Schoof H, Haecker A, Lenhard M, Jurgens G and Laux T (1998) Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell 95: 805-815.
Murai K, Takumi S. Koga H and Ogihara Y (2002) Pistillody, homeotic transformation of stamens into pistil-like structures, caused by nuclear-cytoplasm interaction in wheat. Plant J 29: 169 181.
Paterson AH, Bowers JE, Chapman BA, Peterson DG, Rong J and Wicker TM (2004) Comparative genome analysis of monocots and dicots, toward characterization of angiosperm diversity. Curr Opin Biotech 15: 120-125.
Rojo E, Sharma VK, Kovaleva V, Raikhel NV and Fletcher JC (2002) CLV3 is localized to the extracellular space, where it activates the Arabidopsis CLAVATA stem cell signaling pathway. Plant Cell 14: 969-977.
Schoof H, Lenhard M, Haecker A, Mayer KF, Jurgens G and Laux T (2000) The stem cell population of Arabidopsis shoot meristems in maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell 100: 635-644.
Shannon S and Meekswagner DR (1991) A mutation in the Arabidopsis TFL1 gene affects inflorescence meristem development. Plant Cell 3: 877-892.
Smith HM and Hake S (2003) The interaction of two homeobox genes, BREVIPEDICELLUS and PENNYWISE, regulates internode patterning in the Arabidopsis inflorescence. Plant Cell 15: 1717-1727.
Suzaki T, Sate M, Ashikari M, Miyoshi M, Nagato Y and Hirano HY (2004) The gene FLORAL ORGAN NUMBER1 regulates floral meristem size in rice and encodes a leucine-rich repeat receptor kinase orthologous to Arabidopsis CLAVATA1. Development 131: 5649-5657.
Taguchi-Shiobara F, Yuan Z, Hake S and Jackson D (2001) The fasciated ear2 gene encodes a leucine-rich repeat receptor-like protein that regulates shoot meristem proliferation in maize. Genes Dev 15: 2755-2766.
Taylor JS and Raes J (2004) Duplication and divergence: the evolution of new genes and old ideas. Annual Review Genetics 38:615-643.
Tilney-Bassett RAE (1986). Plant Chimeras. London, Edward Arnold.
Veit B, Schmidt RJ, Hake S and Yanofsky MY (1993) Maize floral development: New genes and old mutants. Plant Cell 5:1205-1215.
Venglat P, Dumonceaux T, Parnell L, Rozwadowski K, Babic V, Keller W, Martienssen RA, Selvaraj G and Datla R (2002) The Arabidopsis BREVIPEDICELLUS gene is an important regulator of pedicel and internode development. Proc Natl Aced Sci USA 99:4730-4735.
Vollbrecht E, Reiser L and Hake S (2000) Shoot meristem size is dependent on inbred background and presence of the maize homeobox gene, knotted1. Development 127: 3161-3172.
Watson JD and Crick FH (1953) Molecular structure of nucleic acids; a structure for deoxyribose nucleic acid. Nature 171: 737-738.
Wolfe KH, Gouy M, Yang YW, Sharp PM and Li WH (1989) Date of the monocot-dicot divergence estimated from chloroplast DNA sequence data. Proc Natl Aced Sci USA 86:6201-625.
Yu J, Hu S, Wang J, Wong GK, Li S, Liu B, Deng Y, Dai L, Zhou Y, Zhang X, Cao M, Liu J, Sun J, Tang J, Chen Y, Huang X, Lin W, Ye C, Tong W, Cong L, Geng J, Han Y, Li L, Li W, Hu G, Li J, Liu Z, Qi Q, Li T, Wang X, Lu H, Wu T, Zhu M, Ni P, Han H, Dong W, Ren X ,Feng X, Cui P, Li X, Wang H, Xu X, Zhai W, Xu Z, Zhang J, He S, Xu J, Zhang K, Zheng X, Dong J, Zeng W, Tao L, Ye J, Tan J, Chen X, He J, Liu D, Tian W, Tian C, Xia H, Bao Q, Li G, Gao H, Cao T, Zhao W, Li P,Chen W, Zhang Y, Hu J, Liu S, Yang J, Zhang G, Xiong Y, Li Z, Mao L, Zhou C, Zhu Z, Chen R, Hao B, Zheng W, Chen S, Guo W, Tao M, Zhu L, Yuan L and Yang H (2002) A draft sequence of the rice genome (Oryza sativa L. ssp. indica). Science 296: 79-92.