BioResource Newsletter Vol.5 No.7
|
|
2009/7/31
| Research and Bioresources No. 3 |
|
 Equation for Developing Novel Wheat Strains by Using Equation for Developing Novel Wheat Strains by Using
Genetic Resources
"(AABBDD)-AA-BB-DD=(aabbdd)"
Toshiki NAKAMURA, Vice Team Leader
National Agricultural Research Center for Tohoku Region
|

|
|
|
|
 Dr. Hitoshi Kihara had authored an article entitled, "Gene symbols for starchy and glutinous characters in cereals" in Seiken-Ziho (27-28:45-47) in 1979. In the article, Dr. Kihara has presented his opinion about the relationship between polyploidy and glutinous and nonglutinous rice. It is well known that mochi, which is a representative of glutinous rice, contains amylose-free endosperm starch, which is composed of amylose and amylopectin. The absence of amylose in mochi is due to the malfunction of a granule-bound starch synthase I (GBSSI or commonly referred to as the waxy (Wx) protein) enzyme, which is responsible for amylose synthesis. Dr. Hitoshi Kihara had authored an article entitled, "Gene symbols for starchy and glutinous characters in cereals" in Seiken-Ziho (27-28:45-47) in 1979. In the article, Dr. Kihara has presented his opinion about the relationship between polyploidy and glutinous and nonglutinous rice. It is well known that mochi, which is a representative of glutinous rice, contains amylose-free endosperm starch, which is composed of amylose and amylopectin. The absence of amylose in mochi is due to the malfunction of a granule-bound starch synthase I (GBSSI or commonly referred to as the waxy (Wx) protein) enzyme, which is responsible for amylose synthesis.
Many mochi strains, in which the GBSSI (Wx) gene was mutated, have been identified in diploid plants※ such as rice, barley, and corn. These mochi strains have been used for the production of improved cultivars and considered important resources for genetics research.
However, mochi strains of hexaploid plants※ such as common wheat, oat, and millet have not yet been reported. As a reason for this, Dr. Kihara suggested that although some genes of an alloploid plant could mutate, it is extremely rare that all the genes responsible for amylose synthesis in the genome of the alloploid plant undergo mutation. This is supported by the fact that although many agriculturally beneficial mutant strains, including mochi mutants, have been developed by the treatment of rice, barley, and corn with mutagens such as Co60※ and EMS※, no such variant of wheat has been produced. In addition, many researchers have reported that the resistance of wheat to mutations is higher than that of diploid plants. However, these results do not imply that wheat genes do not mutate naturally or artificially. In fact, these results indicate that wheat exhibits a higher capacity of accommodating greater number of mutations than diploid plants.
|
| |
※ Diploid (or Hexaploid): cells or organisms that harbor two-fold (or six-fold) pairs of chromosomes in comparison with the base chromosome number.
※ Co60: A radioisotope of cobalt
※ EMS: Ethyl methanesulfonate; a volatile mutagenic agent
|
|
 I came up with the abovementioned idea when I analyzed the relationship between amylose content in endosperm starch in wheat and the Wx protein content. The rationale of my analysis was that although no mochi strains were identified in wheat genetic resources, as expected by Dr. Kihara, many partial waxy mutants in which either 1 or 2 of the 3 homologs (Wx-A1, Wx-B1, and Wx-D1) are mutated were found. Moreover, we recognized that many strains were phenotypically wild-type but genotypically different from the wild-type in terms of their analogous genes. In the light of this finding, I realized that such partial mutants could be used for developing numerous complete mutants by hybridization, even in the case of wheat. It appears to be an obvious solution at present, but this approach was not perceived before. To confirm the authenticity of this idea, we performed the following experiment in 1995: A partial mochi mutant derived from Wx-A1 and Wx-B1 and another partial mochi mutant derived from Wx-D1, both lacking the Wx proteins, were screened from approximately 2,000 strains obtained from within and outside of the country. The F2 plants obtained by the hybridization of these strains completely lacked the Wx proteins, and thus mutants lacking amylose, i.e., the mochi strains, were obtained with a probability of (1/4 × 1/4 × 1/4 = 1/64) (Fig. 1). To explain these results to the general audience, I have formulated the following equation "(AABBDD) - AA - BB - DD = (aabbdd) " (Fig. 2). I came up with the abovementioned idea when I analyzed the relationship between amylose content in endosperm starch in wheat and the Wx protein content. The rationale of my analysis was that although no mochi strains were identified in wheat genetic resources, as expected by Dr. Kihara, many partial waxy mutants in which either 1 or 2 of the 3 homologs (Wx-A1, Wx-B1, and Wx-D1) are mutated were found. Moreover, we recognized that many strains were phenotypically wild-type but genotypically different from the wild-type in terms of their analogous genes. In the light of this finding, I realized that such partial mutants could be used for developing numerous complete mutants by hybridization, even in the case of wheat. It appears to be an obvious solution at present, but this approach was not perceived before. To confirm the authenticity of this idea, we performed the following experiment in 1995: A partial mochi mutant derived from Wx-A1 and Wx-B1 and another partial mochi mutant derived from Wx-D1, both lacking the Wx proteins, were screened from approximately 2,000 strains obtained from within and outside of the country. The F2 plants obtained by the hybridization of these strains completely lacked the Wx proteins, and thus mutants lacking amylose, i.e., the mochi strains, were obtained with a probability of (1/4 × 1/4 × 1/4 = 1/64) (Fig. 1). To explain these results to the general audience, I have formulated the following equation "(AABBDD) - AA - BB - DD = (aabbdd) " (Fig. 2).

|
Fig. 1: Development of waxy wheat. Among the 3 redundant GBSSIs (Wx proteins) found in the endosperm, the GBSSIs derived from the A- and B-genomes are lost in the Kanto 107 strain in Japan and the GBSSI derived from the D-genome is lost in the Hakubi (BaiHuo) strain in China.
The hybridization of these strains produces a mochi strain in the F2 generation, of which endosperm turns mahogany when stained with iodine, with a probability of 1/64 as expected.
|
|
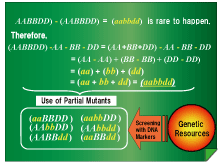
Fig. 2:
The following formula can be applied to develop complete mutants in wheat: (AABBDD) - AA - BB - DD = (aabbdd), where (AABBDD) and (aabbdd) indicate homologs of the A-, B-, and D-genomes of allohexaploid wheat; the upper- and lower-case letters indicate dominant (functioning) and recessive (malfunctioning) genes, respectively; and "-" represents the occurrence of natural or artificial mutations.
|
|
"-(AABBDD) " represents simultaneous mutation of the 3 genes, which, if observed, will be noted as (aabbdd). However, the probability that this event will occur is extremely low. Since each gene is mutually independent, the 3 genes are considered as "(AA + BB + DD)," and the mutation of each gene is then described as "-AA -BB -DD." Hence, the formula can be rewritten as "(AA - AA) + (BB - BB) + (DD - DD)," which is equivalent to "(aa + bb + dd)." In this manner, we screened for mutants in which the homologs were mutated and crossbred these mutant strains.
The probability of producing a full mutant (aabbdd) by this method will be significantly high. However, for the successful realization of this method, it is also important to obtain partial mutants which are not significantly mutated phenotypically but are genotypically different from each other.
|
A successful realization of this formula requires the collection of a wide range of wheat genetic resources that are collected from different places. In addition, it is important to use a convenient and efficient screening method to select mutants which are phenotypically the same but have genotypically different homologs in the A-, B-, and D-genomes. It is important to develop a high-throughput screening method using DNA markers that can identify mutations in the target genes, such as polymorphisms. Genome resources, such as sequence information, will be indispensible for obtaining information about target genes.
Recently, we successfully developed a wheat strain with high amylose content and a sweet wheat strain that accumulates high amounts of maltose, by using DNA markers and the abovementioned formula (Fig. 3). Further, the utility value of the DNA markers used in this method was confirmed to be high for developing novel mutants as well as for improving cultivars for practical aplications.
It is irrefragable that whether the genetic resources essential for the application of the formula can be prepared or not depends on a pure luck or probability. Nevertheless, we screened approximately 200-300 wheat genetic resources obtained from different parts of the world by using polymorphic DNA markers that amplify the coding region of multiple target genes and obtained several mutants in which the homologs were mutated. Furthermore, taken into account the success of developing waxy wheat strains, screening of many wheat strains will raise the possibility of obtaining resources that are necessary for the application of the formula. |
|

Fig. 3: A farm field of the world's first sweet wheat strain (Sweet Wheat) in the flowering season.
If waxy wheat (Fig. 1) and the wheat strain with high amylose content, which lacks the 3 soluble starch synthases (starch synthase IIa enclosed by rectangles) derived from the A-, B-, and D-genomes, are crossbred, a double mutant that completely lacks both the GBSSIs and soluble starch synthases (sweet wheat strain) can be produced with a probability of 1/4096 in the F2 generation.
|
 Recently, practical waxy wheat strains were developed and used to manufacture other products. The development of wheat with novel characteristics will prove to be beneficial for the society, and to achieve this, the genetic resources are considered as gold mines. In keeping with the increase in the amount of genome information in the future, the expansion, maintenance, and administration of genetic resources should also be given more importance. With this perspective, we have great expectations for the advancement of the National BioResource Project (NBRP).■ Recently, practical waxy wheat strains were developed and used to manufacture other products. The development of wheat with novel characteristics will prove to be beneficial for the society, and to achieve this, the genetic resources are considered as gold mines. In keeping with the increase in the amount of genome information in the future, the expansion, maintenance, and administration of genetic resources should also be given more importance. With this perspective, we have great expectations for the advancement of the National BioResource Project (NBRP).■
|
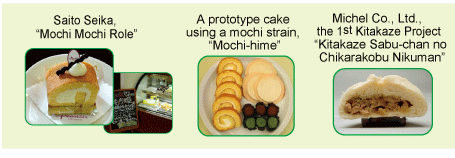
Fig. 4: A product made from Mochi-hime, which is a waxy
wheat strain adapted to the Tohoku region.
|
|
|
|

|
Erudite Lecture Series by Dr. Benno: No. 7 |

|
|
|
Middle-aged men often work on computers for the entire day; in addition, they do not exercise and resort to smoking due to heavy stress levels. These men often eat high-calorie food in a hasty manner and at irregular times. According to an investigation conducted by a pharmaceutical firm, the toilets smell very badly after being used by middle-aged men. In approximately 80% of the cases, the family members claim that the toilet smells very badly after being used by middle-aged men. It is often said that "middle-aged men are stinky," which is actually true.
I used to eat mostly meat until 5 years ago. I have recently switched over to a complete vegetarian diet; however, my family members still claim that the toilet smells very badly after I have used it. I have noticed that when I consume alcohol, meat, or foods containing garlic, my stools give off an extremely bad odor.

|
The odor of stools is attributed to harmful bacteria, such as Clostridium and Clostridium perfringens in the colon. These bacteria act on the food remnants in the colon and produce hazardous substances such as ammonia, indoles, and skatoles that are responsible for the offensive odor.
The odor of flatus can be explained on similar lines. Flatus refers to the expulsion of gases generated during the decomposition of meals by enteric bacteria. Approximately 90% of the flatus comprises odorless gases such as nitrogen, hydrogen, methane, and carbon dioxide, and 10% comprises gases with odor, which is similar to that of the stools. The odor of flatus and stools can be attributed to similar substances such as the hazardous substances produced by harmful bacteria that become predominant in the colon.
|
With age, the bowel movement also becomes slow. The abdominal pressure and muscles are weakened, significantly affecting the bowel movement. This leads to an increase in the number of harmful bacteria and a decrease in the number of beneficial bacteria, facilitating the decomposition of substances in the colon. In addition, the odor of stools becomes stronger and the amount of stool decreases with age. Hence, sometimes, elder people pass granular stool, i.e., "senile (geriatric) narrow stool," after which they might experience a feeling of incomplete evacuation.
Thus far, it has been reported that mostly the elderly tend to pass narrow stools; however, currently many young people have also started passing stools of the similar consistency. The malodorous stools of productive men and the inverse relationship between actual age and colon age may be considered as danger signals. This attributes to the saying, "Suspect diseases when your fart smells."
|

|
| |
|
|
|
 A Visit to the Research Institute for Bioresources, A Visit to the Research Institute for Bioresources,
Okayama University
In the end of May, we visited the Research Institute for Bioresources, Okayama University, which is the core institution of the NBRP on Barley. The Research Institute for Bioresources is located at a 5-min walk from Bikan historical quarter, Kurashiki, where the Ohara Museum of Art is located.
|

|
|
The objective of this visit was to see the barley fields before the harvest. More than 1,600 strains of barley, including those from the NBRP collection, were planted at every 1 meter in the field. It was a beautiful sight where the golden (and some black and green) ears of the barley crop were windswept; watching this sight was indeed a sense of deja vu for us. These fields had a unique collection of strains, including Haruna-Nijo (used for beer production), wild-type barley strains, and domestic barley strains collected from areas all over the world such as East Asia, China, and Africa. In particular, the ear trait was diverse, and there were striking differences between the ears of the Nijo and Rokujo strains; furthermore, the colors (albino, black, or yellow) and the beards (long, short, or none) also varied. |
Some of these fields were being used for research purposes. It is believed that due to the huge genome size of barley, approximately 6,000 individuals of substitution lines are necessary for identifying DNA markers. I participate in developing CMap, a comparative genetic map viewer, of barley and realized the enormous amount of time required for crossbreeding and performing experiments to obtain the data. Next, we were given a tour of the facility where we saw the room used for the drying of ears, refrigerators used for seed storage, freezers used for stocking the BAC clone libraries, and the sequencer (the third of its kind in Japan).
| |

From left to right; Takahashi, Dr. Sato, and Tsuchiya |
The tour took about an hour and a half. We sincerely thank Dr. Sato for his hospitability. In addition, we would like to thank him for sparing time for us during this busy harvest season.
The institute is opened to public visitors in May every year, so please do visit this center to see the barley fields.
(Rie Tsuchiya) |
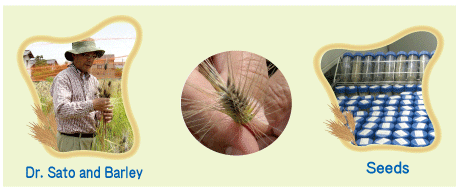 |
At the entrance, we were greeted by the institute logo formed with lawn. The barley fields were open for visitors. Barley strains with different traits were cultivated here. We were amazed by the diversity of the traits and the variety of barley strains. The strains cultivated at this center exhibited novel characteristics such as groveling, purple ears, and bulbs. Approximately 1,500 barley strains are cultivated at this center for the National BioResource Project (NBRP). |

Logo of the RIB |
Subsequently, we were given a tour of the interiors of the facility. We were also shown how the shed seeds are preserved in tubes inside the seed storage. We were informed that the strains distributed by NBRP are preserved in the storage facility. In addition, we saw many other advanced research equipments such as 16 filters containing 300,000 clones and plates with BAC clones.
I would like to encourage people to utilize the Barley Database (http://www.shigen.nig.ac.jp/barley/) that contains the data of barley strains and EST clones.
Finally, I sincerely thank Dr. Sato for giving us a tour of the facility in spite of his busy schedule.
(Yuka Takahashi)
|
|
Editor's Note : Our congratulations to Dr. Nakamura whose paper entitled "Genetic Analysis of Starch Mutations in Wheat and Their Applications in Cross-breeding," received the special prize of the 17th Kihara Memorial Yokohama Foundation. Dr. Nakamura, who is an expert in breeding research, says "The greatest happiness is to see cultivated mochi and sweet wheat strains in the fields both within the country and in other countries." I encourage the readers of this newsletter to recollect the proposed formula when they encounter foods made from "Mochi-hime." It is worrisome how the abnormal weather during this summer will affect the field crops. (Y.Y.)
|
Contact Address:
1111 Yata, Mishima-shi, Shizuoka 411-8540, Japan
Center for Genetic Resource Information, National Institute of Genetics
Tel: 055-981-6885 (Yamazaki)
E-mail brnews@chanko.lab.nig.ac.jp
"translated by ASL translation service and proofread by Sharoh Yip"
|
|



 Dr. Hitoshi Kihara had authored an article entitled, "Gene symbols for starchy and glutinous characters in cereals" in Seiken-Ziho (27-28:45-47) in 1979. In the article, Dr. Kihara has presented his opinion about the relationship between polyploidy and glutinous and nonglutinous rice. It is well known that mochi, which is a representative of glutinous rice, contains amylose-free endosperm starch, which is composed of amylose and amylopectin. The absence of amylose in mochi is due to the malfunction of a granule-bound starch synthase I (GBSSI or commonly referred to as the waxy (Wx) protein) enzyme, which is responsible for amylose synthesis.
Dr. Hitoshi Kihara had authored an article entitled, "Gene symbols for starchy and glutinous characters in cereals" in Seiken-Ziho (27-28:45-47) in 1979. In the article, Dr. Kihara has presented his opinion about the relationship between polyploidy and glutinous and nonglutinous rice. It is well known that mochi, which is a representative of glutinous rice, contains amylose-free endosperm starch, which is composed of amylose and amylopectin. The absence of amylose in mochi is due to the malfunction of a granule-bound starch synthase I (GBSSI or commonly referred to as the waxy (Wx) protein) enzyme, which is responsible for amylose synthesis.










